Abstract
Multiple endocrine neoplasia type 1 (MEN1) is a rare autosomal dominant genetic disorder characterized by neoplasia of the parathyroid, pancreatic islets, and anterior pituitary. In this report, we present a family case in which the proband was diagnosed with prolactinoma 25 years ago. During the current hospitalization, the patient was diagnosed with insulinoma, primary hyperparathyroidism, and adrenocortical carcinoma. The final diagnosis was MEN1, confirmed by identifying a heterozygous mutation in the MEN1 gene through genetic testing. The proband's son also tested positive for the same MEN1 gene mutation, although he exhibited no clinical symptoms. MEN1 associated with adrenocortical carcinoma is exceptionally rare, carries a high malignancy risk, and has a poor prognosis. Genetic testing for the MEN1 gene is crucial for accurate diagnosis, while family screening is beneficial for early detection, timely treatment, and improving patient outcomes.
Keywords: adrenocortical carcinoma, multiple endocrine neoplasia type 1, Cushing syndrome, hyperparathyroidism, gene mutation
Introduction
Multiple endocrine neoplasia type 1 (MEN1) is a rare genetic disorder caused by pathogenic variants in the MEN1 tumor suppressor gene located on chromosome 11q13. This gene is responsible for producing the protein menin, which comprises 610 amino acids and plays a pivotal role in regulating cell transcription, proliferation, and DNA replication and repair processes [1, 2]. As a tumor suppressor, MEN1 is crucial for maintaining cellular growth control. However, pathogenic variants leading to the dysfunction or inactivation of the menin protein can disrupt this balance, allowing cells to proliferate unchecked and ultimately giving rise to tumor development.
Adrenocortical carcinoma (ACC) is a rare endocrine malignancy characterized by high aggressiveness, rapid progression, a tendency for local metastasis, and a low overall survival rate. In adults, most cases of ACC are sporadic, but there is a genetic predisposition in about 10% of cases, often occurring as part of inherited syndromes such as MEN1 [1]. The incidence of ACC in MEN1 patients is rarely reported.
Here, we review 25 years of complete case data for a patient with MEN1 who was initially diagnosed with a pituitary tumor and later developed adrenocortical carcinoma. By integrating family analysis, our review aims to facilitate early diagnosis and treatment for individuals carrying gene mutations while enhancing the overall understanding of this disease.
Case Presentation
A 49-year-old female farmer was admitted to the hospital presenting with symptoms of hypoglycemia. Twenty-five years earlier, she had been diagnosed with pituitary macroprolactinoma, underwent pituitary tumor resection, and recovered after surgery. Since then, the patient had not visited the hospital for follow-up. Two years previously, the patient presented with recurrent palpitations, diaphoresis, fatigue, and weight gain, leading to a diagnosis of hypoglycemia. The lowest recorded blood sugar level was 1.0 mmol/L (18 mg/dL) (reference range, 3.9-6.1 mmol/L [70-109 mg/dL]), which could be alleviated by food intake. Despite thorough examination at the local hospital, the etiology of hypoglycemia remained elusive, subsequently leading to manifestations of taciturnity and alterations in personality. She had 2 sons, and her family members had not been diagnosed with any particular inherited disorders.
Diagnostic Assessment
The patient presented with stable vital signs (temperature: 36.2 °C, pulse: 115 per minute, respiratory rate: 18 per minute, blood pressure: 122/87 mmHg) and a body mass index (BMI) of 29.34 kg/m2. However, physical examination revealed distinct features, including centripetal obesity, moon face, and buffalo hump. The patient experienced repeated episodes of fasting hypoglycemia in the hospital (Table 1). Contrast-enhanced computed tomography (CT) scan showed a pancreatic mass suggestive of a possible endocrine tumor (Fig. 1A). Considering the patient's hypoglycemia, hyperinsulinism, and presence of a pancreatic mass in conjunction, a diagnosis of insulinoma was established. Physical examination revealed signs of Cushing syndrome, and the cortisol rhythm was further evaluated, which indicated non-adrenocorticotropic hormone (ACTH)-dependent hypercortisolism (Table 2). An enhanced abdominal CT scan revealed a huge mass in the left adrenal region (Fig. 1B). Dehydroepiandrosterone sulfate (DHEA-S) and plasma metanephrine were within the reference range on preoperative evaluation. Given the patient's history of pituitary surgery and signs of polyglandular disease, various hormonal examinations were conducted (Table 3). Combined with high calcium, low phosphorus, and elevated parathyroid hormone (PTH), Doppler ultrasound indicated parathyroid adenoma, which was diagnosed with primary hyperparathyroidism. Meanwhile, quantitative computed tomography (QCT) bone mineral density was 69.7 mg/cm³, suggesting osteoporosis. Abdominal CT showed multiple stones in both kidneys and ureters with hydronephrosis. The level of serum creatinine was 130.9 μmol/L (1.48 mg/dL) (reference range, 49-90 μmol/L [0.55-1 mg/dL]). To confirm the diagnosis, a gallium-68-labeled 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid-d-Phel-Tyr3-Thr8-OC (68Ga-DOTATATE) positron emission tomography/computed tomography (PET/CT) scan was performed, which revealed bilateral parathyroid masses, multiple pancreatic masses, and a large mass in the left adrenal gland. All of these masses exhibited high expression of somatostatin receptors. (Figure 2A and 2B). Considering the patient's medical history, laboratory examination, and 68Ga-DOTATATE PET/CT findings, the results were consistent with the indications of multiple endocrine neoplasia. Concurrently, genetic testing revealed a mutation in the MEN1 gene, specifically c.333 (exon 2)_c.334 (exon 2) insT (Fig. 3).
Table 1.
Hypoglycemia and simultaneous insulin, C-peptide
| Date | Blood glucose | β-hydroxybutyrate | Insulin | C-peptide |
|---|---|---|---|---|
| Day 1 | 2.33 mmol/L (42 mg/dL) | 0.05 mmol/L (0.52 mg/dL) | 149 pmol/L (21.4 μIU/mL) | 2.49 nmol/L (7.5 ng/mL) |
| Day 2 | 2.9 mmol/L (52 mg/dL) | 0.05 mmol/L (0.52 mg/dL) | 164 pmol/L (23.67 μIU/mL) | 2.79 nmol/L (8.4 ng/mL) |
| Day 3 | 2.67 mmol/L (48 mg/dL) | 0.05 mmol/L (0.52 mg/dL) | 156 pmol/L (22.44 μIU/mL) | 2.44 nmol/L (7.4 ng/mL) |
| Day 4 | 1.96 mmol/L (35 mg/dL) | 0.04 mmol/L (0.4 mg/dL) | 163 pmol/L (23.44 μIU/mL) | 2.26 nmol/L (6.8 ng/mL) |
| Reference | 3.9-6.1 mol/L (70-109 g/dL) | 0.02-0.27 mmol/L (0.2-2.8 mg/dL) | 10.4-104 mol/L (1.5-15 μIU/mL) | 0.3-1.11 nmol/L (0.9-3 ng/mL) |
Figure 1.
Abdominal CT scan findings. (A) Abnormal enhancement of the neck of pancreas (19 mm × 16 mm), suggestive of a possible endocrine tumor. (B) Left upper abdominal mass (85 mm × 81 mm × 87 mm), raising the possibility of a gastric stromal tumor, left adrenal pheochromocytoma, or other.
Table 2.
Cortisol rhythm and LDDST
| 8am | 4pm | 0:00 | LDDST (8am) | |
|---|---|---|---|---|
| Cortisol | 464.4 nmol/L (16.8 μg/dL) | 453.3 nmol/L (16.4 μg/dL) | 359.3 nmol/L (13 μg/dL) | 453.3 nmol/L (16.4 μg/dL) |
| ACTH | 0.39 pmol/L (1.76 pg/mL) | 0.25 pmol/L (1.14 pg/mL) | 0.22 pmol/L (1.01 pg/mL) | <0.22 pmol/L (<1 pg/mL) |
| 24h UFC: 2684 nmol/24 hours (97.3 μg/24 hours) (Reference, 97-1241 nmol/24 hours [3.5-45 μg/24 hours]) | ||||
Abbreviations: ACTH, adrenocorticotropin; LDDST, low-dose dexamethasone suppression test; UFC, urinary free cortisol.
Table 3.
Laboratory values
| Test | Result | Reference range |
|---|---|---|
| Potassium | 2.9 mmol/L (2.9 mEq/L) | 3.5-5.3 mmol/L (3.5-5.5 mEq/L) |
| 24 hours urine potassium | 57.07 mmol/24h (57.07 mEq/24 hours) | |
| Calcium | 2.98 mmol/L (11.9 mg/dL) | 2.21-2.81 mmol/L (8.9-11.3 mg/dL) |
| Phosphorus | 0.62 mmol/L (1.9 mg/dL) | 0.81-1.51 mmol/L (2.5-4.7 mg/dL) |
| PTH | 162 pmol/L (1535 pg/mL) | 1.6-6.9 pmol/L (15-65 pg/mL) |
| FSH | 2.26 mIU/mL (2.26 IU/L) | 26.72-133.41 mIU/mL (26.72-133.41 IU/L) |
| LH | 0.49 mIU/mL (0.49 IU/L) | 0.38-1.97 mIU/mL (0.38-1.97 IU/L) |
| E2 | 146.9 pmol/L (40 pg/mL) | <36.7 pmol/L (<10 pg/mL) |
| T | 2.18 nmol/L (0.6 ng/mL) | 0.38-1.97 nmol/L (0.1-0.6 ng/mL) |
| DHEA-S | 0.45 μmol/L (16 μg/dL) | 0.44-6.47 μmol/L (16-238 μg/dL) |
| PRL | 8.32 μg/L (8.32 ng/mL) | 5.18-26.53 μg/L (5.18-26.53 ng/mL) |
| IGF-1 | 10.9 nmol/L (82.8 ng/mL) | 6.8-42.9 nmol/L (52-328 ng/mL) |
| ARR | 4.48 | <30 |
| Plasma MN | 0.06 nmol/L (12.18 pg/mL) | 0-0.5 nmol/L (0-100 pg/mL) |
| Plasma NMN | <0.08 nmol/L (<15 pg/mL) | 0-0.96 nmol/L (0-160 pg/mL) |
| G-17 | 6.44 pmol/L (13.5 pg/mL) | 1-7 pmol/L (2.1-14.7 pg/mL) |
| TSH | 0.33 mIU/L (0.33 IU/L) | 0.35-4.94 mIU/L (0.35-4.94 IU/L) |
| FT4 | 8.69 pmol/L (0.67 ng/dL) | 9.01-19.05 pmol/L (0.7-1.5 ng/dL) |
| 25-(OH)Vit-D | 26.76 nmol/L (10.7 ng/mL) | >75 nmol/L (>30 ng/mL) |
| P1NP | 157.1 ng/mL (157.1 μg/L) | 21.32-112.8 ng/mL (21.32-112.8 μg/L) |
| β-CTX | 1.75 ng/mL (1.75 μg/L) | 0.131-0.9 ng/mL (0.131-0.9 μg/L) |
| Diabetes autoantibodies (GAD, IAA, and ICA): negative | ||
Abbreviations: ARR, aldosterone renin ratio; β-CTX, β-carboxyterminal crosslinked telopeptide of type 1 collagen; DHEA-S, dehydroepiandrosterone sulfate; E2, estradiol; FSH, follicle-stimulating hormone; FT4, free thyroxine; G-17, gastrin-17; IGF-1, insulin-like growth factor 1; LH, luteinizing hormone; MN, metanephrine; NMN, normetanephrine; P1NP, N-terminal propeptide of type I procollagen; PRL, prolactin; PTH, parathyroid hormone; T, testosterone; TSH, thyroid-stimulating hormone.
Figure 2.
68Ga-DOTATATE PET/CT imaging findings. The PET/CT scan revealed. (A) a right parathyroid lesion measuring 22 mm × 10 mm (SUV4.1), (B) showing a left adrenal mass measuring 89 mm × 94 mm (SUV6.2) and multiple lesions of pancreas with the largest area measuring about 18 mm (SUV33.7).
Figure 3.
MEN1 gene detection in the proband. Note: Gene detection showed MEN1 heterozygous mutation c.333 (exon2)_c.334(exon2) insT.
Treatment
Following a comprehensive discussion with a multidisciplinary team, the patient underwent ureteral lithotripsy and right ureteral stent implantation to solve urinary obstruction and relieve obstructive nephropathy. Furosemide and salmon calcitonin were used to treat hypercalcemia preoperatively. Next, the urological and pancreatic surgeons combined to perform a left adrenalectomy and distal pancreatectomy. Pathological findings revealed multiple pancreatic neuroendocrine neoplasms, with diameters ranging from 5 to 22 mm, consistent with G1 and G2 neuroendocrine neoplasms (Fig. 4). The left adrenal tumor exhibited extensive necrosis, which was considered mucoid adrenocortical carcinoma and measured 105 mm × 92 mm × 63 mm (Fig. 5). The patient's blood glucose normalized postoperatively. Low-molecular-weight heparins were administered for anticoagulation, and glucocorticoids were used to prevent postoperative adrenal insufficiency. She was expected to be operated on for parathyroidectomy once her condition stabilized.
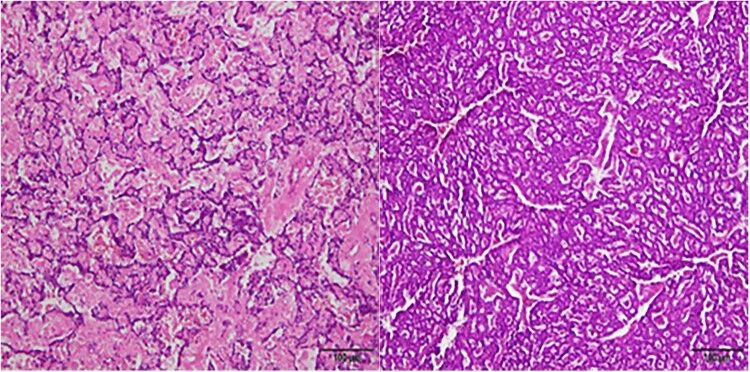
Figure 4.
Pathological findings of the pancreas. Multiple neuroendocrine tumors were consistent with G1 and G2 changes with mitotic signs < 2/10HPF. Immunohistochemical results: CgA+, Syn+, SSTR2 (part 1+, Part 3+), P53 wild type, Ki67 (2-4%).
Figure 5.
Pathological findings of the adrenal gland. The left adrenal tumor was identified as mucinous adrenocortical carcinoma, exhibiting mitotic signs > 5/50HPF and capsular invasion, without venous invasion or sinusoidal invasion. Immunohistochemical results: PCK-, CR-, MelanA±, Vim+, Syn+, CgA-, S-100-, HMB45±, β-catenin + (membrane), SOX-10-, P53wild type, Ki67+ (15%), SF1(−).
Outcome and Follow-Up
After undergoing adrenalectomy and pancreatectomy, the patient developed complications, including an abdominal abscess and intestinal obstruction, despite receiving treatment with the antibiotic meropenem. As a result, she underwent intestinal resection and drainage of the abdominal abscess. The patient experienced significant complications, including severe sepsis and septic shock, prompting her family to discontinue treatment. Consequently, the patient died. Subsequent family investigation (Fig. 6) unveiled that one of her sons carried the same MEN1 mutation gene, yet clinical screenings did not detect any symptoms associated with MEN1.
Figure 6.
Family tree. Note: proband; MEN1 mutation carrier.
Discussion
We present a case of a MEN1 patient diagnosed with ACC, in whom genetic testing identified a heterozygous mutation of the MEN1 gene (c.333(exon2)_c.334(exon2) insT), a rare endocrine disorder. Family analysis revealed that the patient's son also carried the same pathogenic gene and remained asymptomatic.
MEN1 is a rare autosomal dominant genetic disorder with a prevalence of 3 to 10 per 100 000 [2]. Patients with MEN1 present with various clinical manifestations, which may be related to different mutation sites and types. MEN1 can involve more than 30 endocrine glands/organs in the body, with varying penetrance of each tumor [3]. Among them, the prevalence of primary parathyroid adenoma is the highest. MEN1 patients often have multiple tumors, and the corresponding tumors may be larger and more invasive compared to non-MEN1 patients. They are usually discovered at a more advanced stage with distant metastases, leading to poor treatment outcomes and a shorter life expectancy [4-6].
ACC, a sporadic and highly aggressive malignant tumor, is relatively rare in MEN1, with a reported incidence of 0.5 to 2 cases per million populations annually. Patients typically have a poor prognosis, with a 5-year overall survival rate of only 16% to 44% [7, 8]. ACC can occur at all ages, but the mean age of diagnosis in MEN1 is younger than that of sporadic ACC. Overall, approximately 10% of ACC cases occur in patients with a genetic predisposition, so it is recommended that all ACC patients undergo clinical genetic testing. ACC has been reported as part of several hereditary cancer susceptibility syndromes [8], such as MEN1, Li-Fraumeni syndrome, Lynch syndrome, Beckwith-Wiedemann syndrome, and familial adenomatous polyposis. Studies suggested that the molecular pathogenesis of ACC mainly involves gene mutations (CCND1, TOP2A, TP53, etc.) and the abnormal expressions of steroidogenic factor 1 (SF-1), insulin-like growth factor 2 (IGF-2), and vascular endothelial growth factor (VEGF). Abnormal activation of the Wnt signaling pathway, microRNA dysregulation, and DNA methylation have also been associated with the occurrence and development of ACC. It has been reported that the incidence of ACC in patients with MEN1 is approximately 2.6% to 6%, and the prevalence of ACC in patients with MEN1 and concurrent adrenal tumors is about 10 times higher than in patients with sporadic adrenal incidentalomas without MEN1 [9, 10]. In specific cohorts, the incidence of ACC in MEN1-related adrenal tumors can be as high as 13.8% [11].
At present, there are no unequivocal tumor markers for ACC. Nonetheless, detecting SF-1, Melan-A, and inhibin-A via immunohistochemical tests can aid in determining the source of adrenal tumors. The patient had been assigned a Weiss score of 3 and a Ki67 proliferation index of 15%, leading to a definitive diagnosis of ACC. Ki67 is the main pathological prognostic factor in ACC patients. The higher the level of Ki67, the worse the prognosis. Ki67 > 10% indicates a high risk of postoperative recurrence. Surgical resection is the preferred treatment for ACC patients, especially for those classified within stages I to III of the European Network for the Study of Adrenal Tumors (ENSAT) staging. The patient was at ENSAT stage III and presented with a high Ki67 index, warranting postoperative adjuvant therapy with mitotane. Unfortunately, the patient died due to postoperative complications and a severe infection.
In summary, we presented a case of ACC combined with MEN1 in a patient, representing an uncommon endocrine disorder. Despite undergoing surgical intervention, the patient's concurrent Cushing syndrome posed a significant risk for postoperative infection, ultimately leading to fatal complications. Genetic testing revealed a heterozygous mutation in the MEN1 gene for this individual. Genetic mutation analysis of 8 family members (6 siblings and 2 sons of the patient) showed that 1 of the sons (11 years old) carries the MEN1 gene mutation. Comprehensive examinations, including blood calcium levels, PTH levels, thyroid function tests, cortisol levels, ACTH levels, sex hormone evaluation, chest and whole abdomen CT scans, and pituitary magnetic resonance imaging (MRI), were conducted on this child but did not reveal any abnormalities. Patients with MEN1 syndrome should undergo clinical and biochemical screening every 6 to 12 months, while radiological screening of the pancreas, adrenal glands, and pituitary using magnetic MRI or CT should be done every 12-36 months. Despite the absence of any functional or morphological abnormalities in her son's endocrine glands at present, a study involving 12 children under 20 years of age from MEN1 families reported that more than 40% of the children will develop one or more MEN1-related tumors [12]. Therefore, it is recommended that individuals carrying MEN1 mutations undergo annual biochemical screening after baseline pituitary and abdominal imaging, and such screenings are repeated every 1 to 3 years. The implementation of regular screening programs has significantly contributed to the diagnosis of MEN1 and early detection of tumors.
Learning Points
ACC exhibits characteristics of both malignant and endocrine tumors, often resulting in adrenal hormone secretion and subsequent development of Cushing syndrome and virilization. Alternatively, it can manifest as a nonfunctional upper abdominal mass with excessive tumor growth or local mass effects. Therefore, a comprehensive hormonal evaluation is essential for any adrenal mass.
ACC is a rare component of MEN1, and nonfunctional adrenal lesions in MEN1 patients have the potential risk of developing ACC. Therefore, regular follow-up should be conducted for MEN1 with adrenal occupation.
Screening MEN1 patients comprehensively and conducting routine clinical follow-ups for asymptomatic carriers is crucial. This approach enables early detection and diagnosis of the disease, leading to improved patient outcomes.
Contributors
All authors made individual contributions to authorship. M.Y. and S.L. conceptualized and designed the case report, gathered and analyzed the data, and drafted the initial and final manuscript. X.Z. contributed to the diagnosis and management of this patient. All authors reviewed and approved the final draft.
Abbreviations
- ACC
adrenocortical carcinoma
- ACTH
adrenocorticotropic hormone
- CT
computed tomography
- MEN1
multiple endocrine neoplasia type 1
- MRI
magnetic resonance imaging
- PET/CT
positron emission tomography/computed tomography
- PTH
parathyroid hormone
- SF-1
steroidogenic factor 1
Contributor Information
Mei Yang, Department of Endocrinology and Metabolism, The Third People's Hospital of Chengdu, Chengdu 610031, China.
Sha Li, Department of Endocrinology and Metabolism, The Third People's Hospital of Chengdu, Chengdu 610031, China.
Xiao Wei Zhong, Department of Endocrinology and Metabolism, The Third People's Hospital of Chengdu, Chengdu 610031, China.
Funding
This study is not funded by any organization.
Disclosures
The authors declare that they have no conflict of interest.
Informed Patient Consent for Publication
Signed informed consent obtained directly from the patient's relatives or guardians.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Else T, Kim AC, Sabolch A, et al. Adrenocortical carcinoma. Endocr Rev. 2014;35(2):282‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goudet P, Cadiot G, Barlier A, et al. French guidelines from the GTE, AFCE and ENDOCAN-RENATEN (Groupe d'étude des Tumeurs Endocrines/Association Francophone de Chirurgie Endocrinienne/Reseau national de prise en charge des tumeurs endocrines) for the screening, diagnosis and management of multiple endocrine neoplasia type 1. Ann Endocrinol (Paris). 2024;85(1):2‐19. [DOI] [PubMed] [Google Scholar]
- 3. Giusti F, Cianferotti L, Boaretto F, et al. Multiple endocrine neoplasia syndrome type 1: institution, management, and data analysis of a nationwide multicenter patient database. Endocrine. 2017;58(2):349‐359. [DOI] [PubMed] [Google Scholar]
- 4. Rogoziński D, Gilis-Januszewska A, Skalniak A, Kluczyński Ł, Pantofliński J, Hubalewska-Dydejczyk A. Pituitary tumours in MEN1 syndrome—the new insight into the diagnosis and treatment. Endokrynol Pol. 2019;70(5):445‐452. [DOI] [PubMed] [Google Scholar]
- 5. Trouillas J, Labat-Moleur F, Sturm N, et al. Pituitary tumors and hyperplasia in multiple endocrine neoplasia type 1 syndrome (MEN1): a case-control study in a series of 77 patients versus 2509 non-MEN1 patients. Am J Surg Pathol. 2008;32(4):534‐543. [DOI] [PubMed] [Google Scholar]
- 6. Sakurai A, Yamazaki M, Suzuki S, et al. Clinical features of insulinoma in patients with multiple endocrine neoplasia type 1: analysis of the database of the MEN Consortium of Japan. Endocr J. 2012;59(10):859‐866. [DOI] [PubMed] [Google Scholar]
- 7. Fassnacht M, Dekkers OM, Else T, et al. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2018;179(4):G1‐G46. [DOI] [PubMed] [Google Scholar]
- 8. Lerario AM, Moraitis A, Hammer GD. Genetics and epigenetics of adrenocortical tumors. Mol Cell Endocrinol. 2014;386(1-2):67‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Griniatsos JE, Dimitriou N, Zilos A, et al. Bilateral adrenocortical carcinoma in a patient with multiple endocrine neoplasia type 1 (MEN1) and a novel mutation in the MEN1 gene. World J Surg Oncol. 2011;9:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gatta-Cherifi B, Chabre O, Murat A, et al. Adrenal involvement in MEN1. Analysis of 715 cases from the Groupe d'etude des Tumeurs Endocrines database. Eur J Endocrinol. 2012;166(2):269‐279. [DOI] [PubMed] [Google Scholar]
- 11. Wang W, Han R, Ye L, et al. Adrenocortical carcinoma in patients with MEN1: a kindred report and review of the literature. Endocr Connect. 2019;8(3):230‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Newey PJ, Jeyabalan J, Walls GV, et al. Asymptomatic children with multiple endocrine neoplasia type 1 mutations may harbor nonfunctioning pancreatic neuroendocrine tumors. J Clin Endocrinol Metab. 2009;94(10):3640‐3646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.