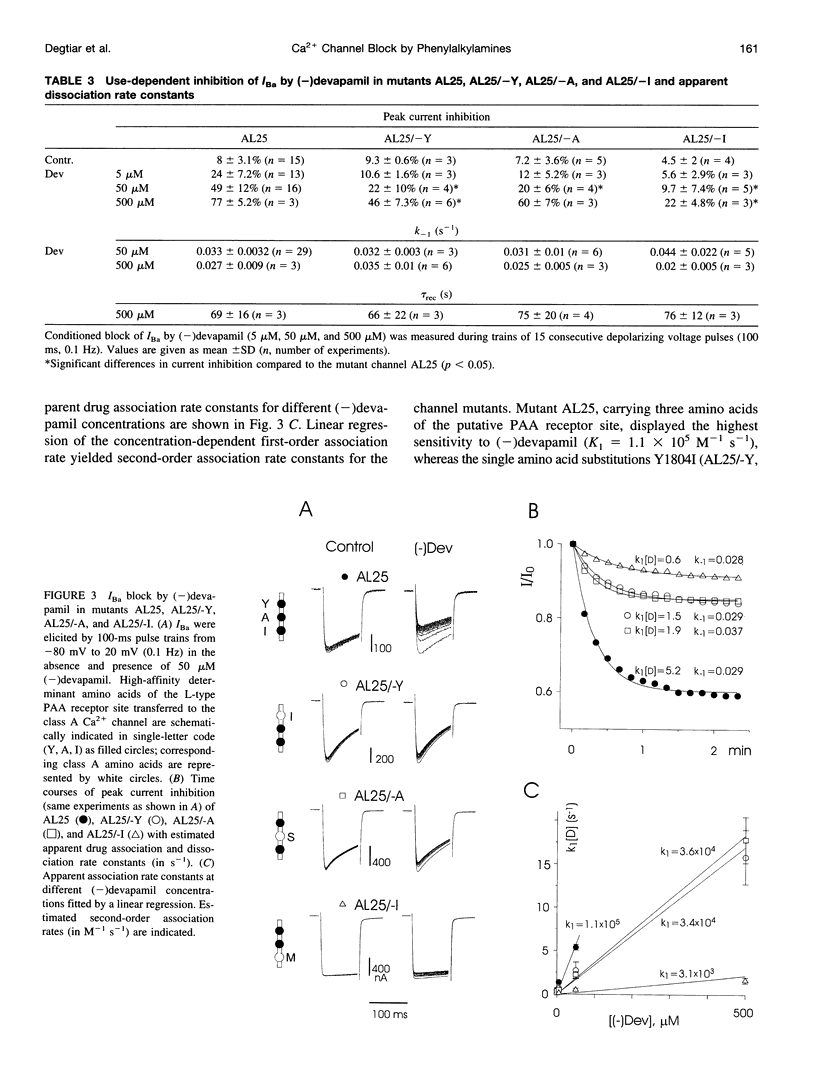
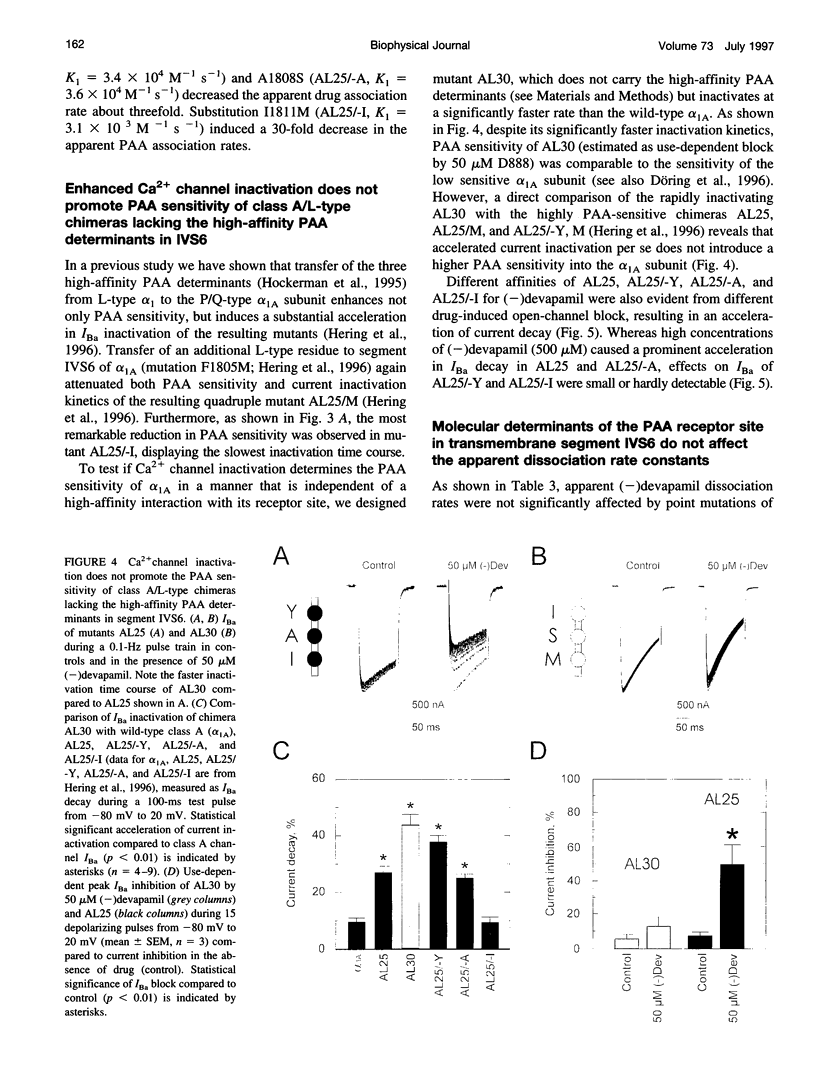
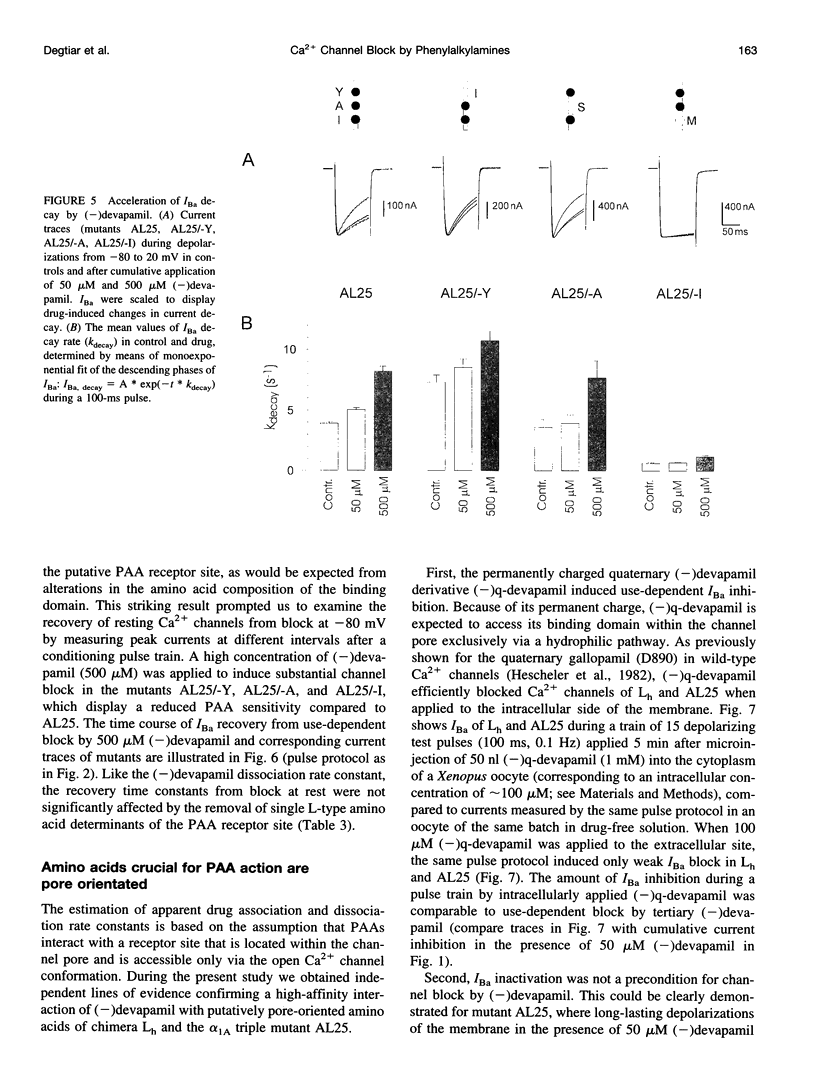
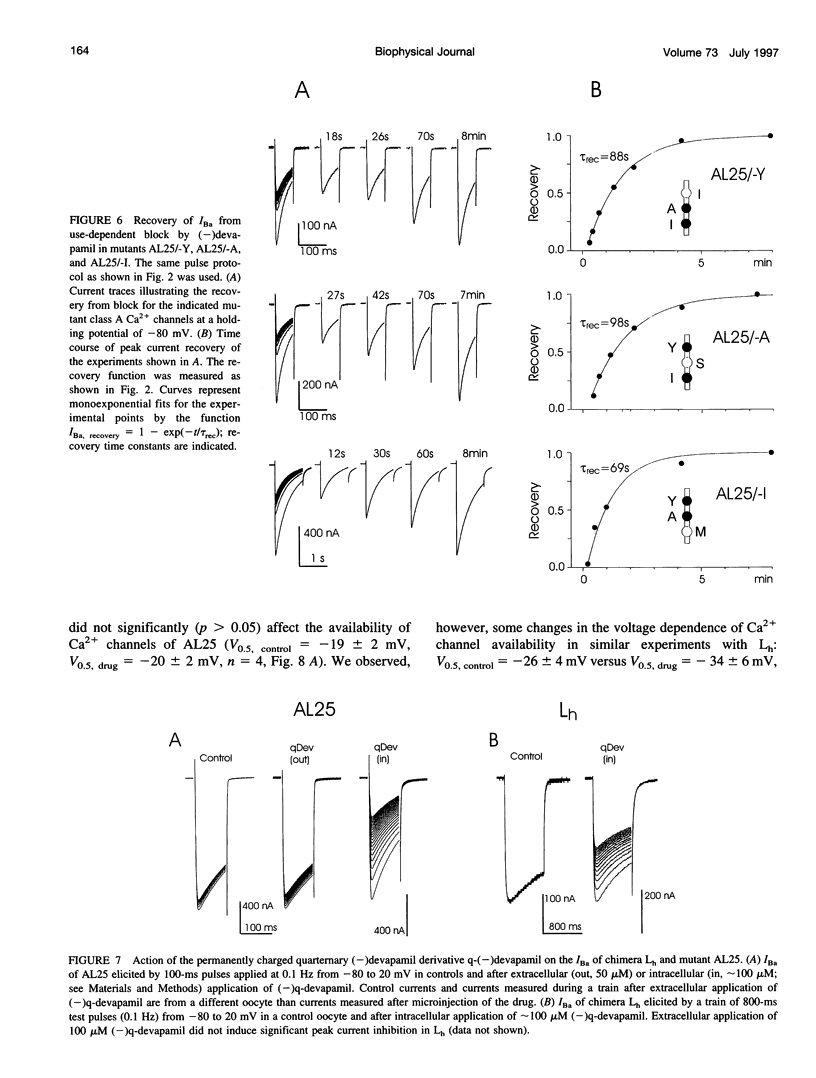
Abstract
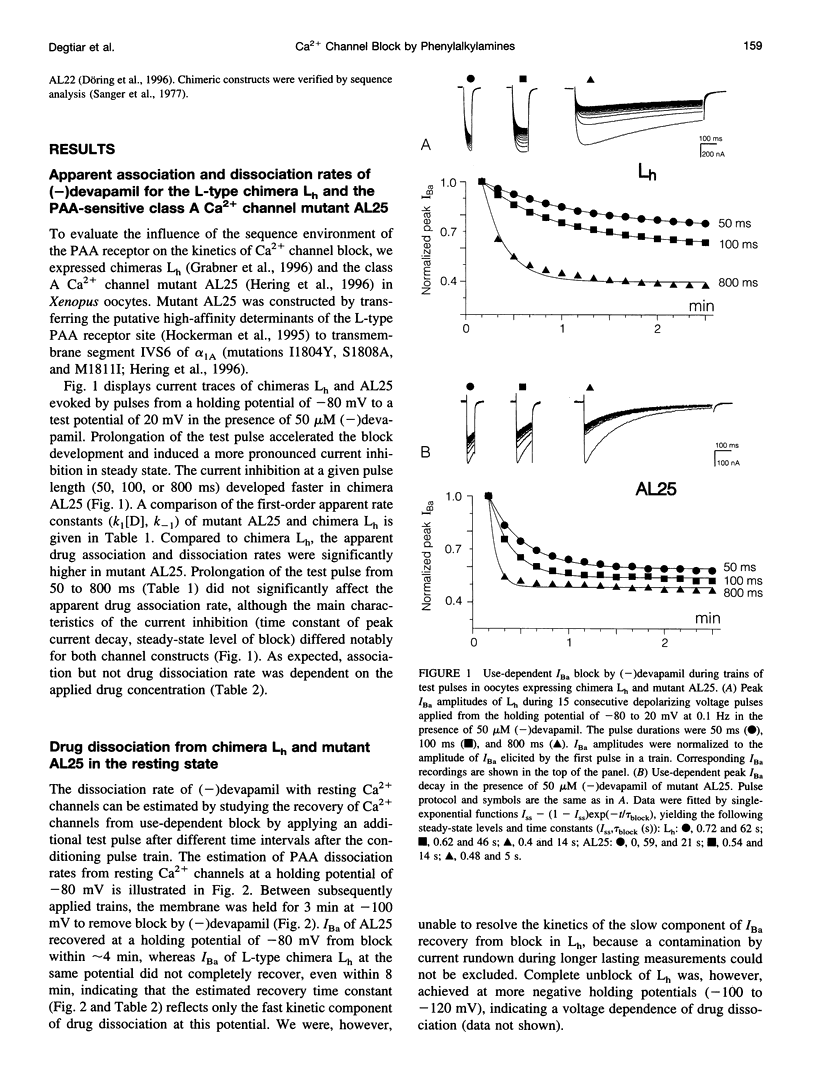
The pore-forming alpha 1 subunit of L-type calcium (Ca2+) channels is the molecular target of Ca2+ channel blockers such as phenylalkylamines (PAAs). Association and dissociation rates of (-)devapamil were compared for a highly PAA-sensitive L-type Ca2+ channel chimera (Lh) and various class A Ca2+ channel mutants. These mutants carry the high-affinity determinants of the PAA receptor site in a class A sequence environment. Apparent drug association and dissociation rate constants were significantly affected by the sequence environment (class A or L-type) of the PAA receptor site. Single point mutations affecting the high-affinity determinants in segments IVS6 of the PAA receptor site, introduced into a class A environment, reduced the apparent drug association rates. Mutation I1811M in transmembrane segment IVS6 (mutant AL25/-I) had the highest impact and decreased the apparent association rate for (-)devapamil by approximately 30-fold, suggesting that this pore-lining isoleucine in transmembrane segment IVS6 plays a key role in the formation of the PAA receptor site. In contrast, apparent drug dissociation rates of Ca2+ channels in the resting state were almost unaffected by point mutations of the PAA receptor site.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean B. P. Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6388–6392. doi: 10.1073/pnas.81.20.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaumer L., Campbell K. P., Catterall W. A., Harpold M. M., Hofmann F., Horne W. A., Mori Y., Schwartz A., Snutch T. P., Tanabe T. The naming of voltage-gated calcium channels. Neuron. 1994 Sep;13(3):505–506. doi: 10.1016/0896-6273(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Catterall W. A., Striessnig J. Receptor sites for Ca2+ channel antagonists. Trends Pharmacol Sci. 1992 Jun;13(6):256–262. doi: 10.1016/0165-6147(92)90079-l. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Structure and function of voltage-gated ion channels. Annu Rev Biochem. 1995;64:493–531. doi: 10.1146/annurev.bi.64.070195.002425. [DOI] [PubMed] [Google Scholar]
- Diochot S., Richard S., Baldy-Moulinier M., Nargeot J., Valmier J. Dihydropyridines, phenylalkylamines and benzothiazepines block N-, P/Q- and R-type calcium currents. Pflugers Arch. 1995 Nov;431(1):10–19. doi: 10.1007/BF00374372. [DOI] [PubMed] [Google Scholar]
- Dunlap K., Luebke J. I., Turner T. J. Exocytotic Ca2+ channels in mammalian central neurons. Trends Neurosci. 1995 Feb;18(2):89–98. [PubMed] [Google Scholar]
- Döring F., Degtiar V. E., Grabner M., Striessnig J., Hering S., Glossman H. Transfer of L-type calcium channel IVS6 segment increases phenylalkylamine sensitivity of alpha1A. J Biol Chem. 1996 May 17;271(20):11745–11749. doi: 10.1074/jbc.271.20.11745. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Striessnig J. Molecular properties of calcium channels. Rev Physiol Biochem Pharmacol. 1990;114:1–105. doi: 10.1007/BFb0031018. [DOI] [PubMed] [Google Scholar]
- Grabner M., Friedrich K., Knaus H. G., Striessnig J., Scheffauer F., Staudinger R., Koch W. J., Schwartz A., Glossmann H. Calcium channels from Cyprinus carpio skeletal muscle. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):727–731. doi: 10.1073/pnas.88.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabner M., Wang Z., Hering S., Striessnig J., Glossmann H. Transfer of 1,4-dihydropyridine sensitivity from L-type to class A (BI) calcium channels. Neuron. 1996 Jan;16(1):207–218. doi: 10.1016/s0896-6273(00)80037-9. [DOI] [PubMed] [Google Scholar]
- Hering S., Aczél S., Grabner M., Döring F., Berjukow S., Mitterdorfer J., Sinnegger M. J., Striessnig J., Degtiar V. E., Wang Z. Transfer of high sensitivity for benzothiazepines from L-type to class A (BI) calcium channels. J Biol Chem. 1996 Oct 4;271(40):24471–24475. doi: 10.1074/jbc.271.40.24471. [DOI] [PubMed] [Google Scholar]
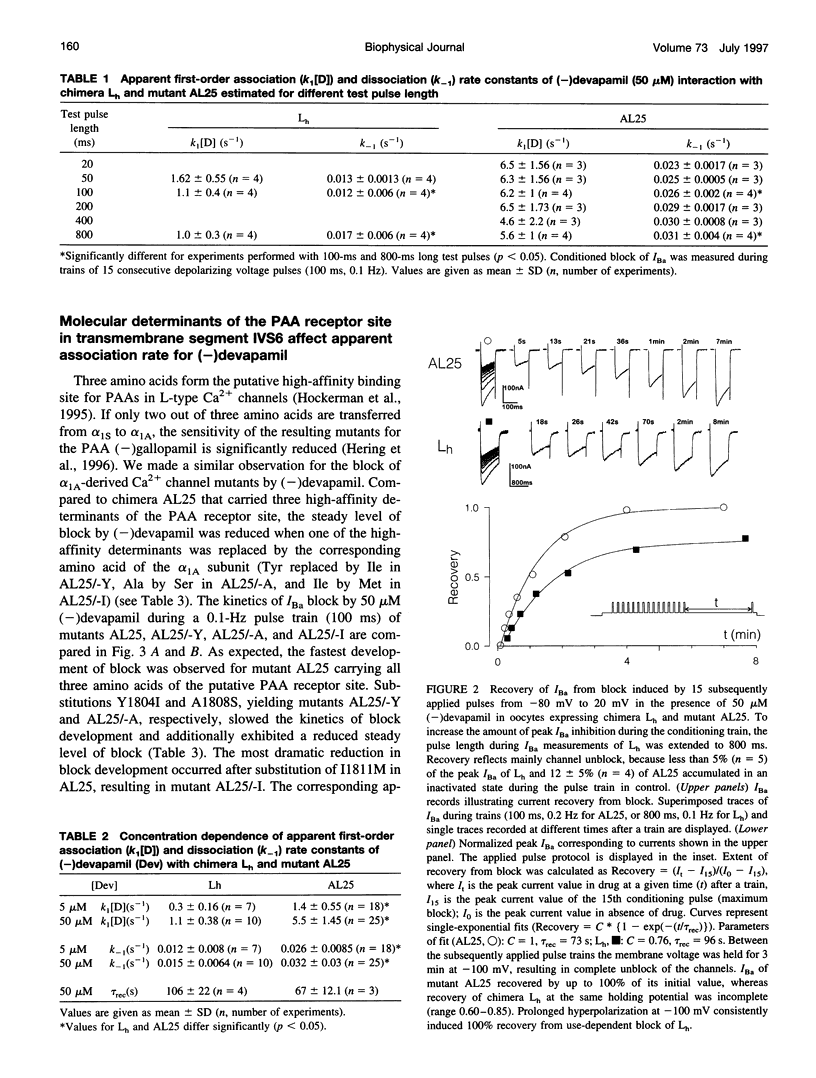
- Hering S., Bolton T. B., Beech D. J., Lim S. P. Mechanism of calcium channel block by D600 in single smooth muscle cells from rabbit ear artery. Circ Res. 1989 May;64(5):928–936. doi: 10.1161/01.res.64.5.928. [DOI] [PubMed] [Google Scholar]
- Hering S., Hughes A. D., Timin E. N., Bolton T. B. Modulation of calcium channels in arterial smooth muscle cells by dihydropyridine enantiomers. J Gen Physiol. 1993 Mar;101(3):393–410. doi: 10.1085/jgp.101.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hescheler J., Pelzer D., Trube G., Trautwein W. Does the organic calcium channel blocker D600 act from inside or outside on the cardiac cell membrane? Pflugers Arch. 1982 Jun;393(4):287–291. doi: 10.1007/BF00581411. [DOI] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977 Apr;69(4):497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockerman G. H., Johnson B. D., Scheuer T., Catterall W. A. Molecular determinants of high affinity phenylalkylamine block of L-type calcium channels. J Biol Chem. 1995 Sep 22;270(38):22119–22122. doi: 10.1074/jbc.270.38.22119. [DOI] [PubMed] [Google Scholar]
- Hofmann F., Biel M., Flockerzi V. Molecular basis for Ca2+ channel diversity. Annu Rev Neurosci. 1994;17:399–418. doi: 10.1146/annurev.ne.17.030194.002151. [DOI] [PubMed] [Google Scholar]
- Horton R. M., Hunt H. D., Ho S. N., Pullen J. K., Pease L. R. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989 Apr 15;77(1):61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- Ishibashi H., Yatani A., Akaike N. Block of P-type Ca2+ channels in freshly dissociated rat cerebellar Purkinje neurons by diltiazem and verapamil. Brain Res. 1995 Oct 9;695(1):88–91. doi: 10.1016/0006-8993(95)00815-8. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature. 1983 Apr 28;302(5911):790–794. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- McDonald T. F., Pelzer D., Trautwein W. Cat ventricular muscle treated with D600: characteristics of calcium channel block and unblock. J Physiol. 1984 Jul;352:217–241. doi: 10.1113/jphysiol.1984.sp015288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami A., Imoto K., Tanabe T., Niidome T., Mori Y., Takeshima H., Narumiya S., Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989 Jul 20;340(6230):230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- Mori Y., Friedrich T., Kim M. S., Mikami A., Nakai J., Ruth P., Bosse E., Hofmann F., Flockerzi V., Furuichi T. Primary structure and functional expression from complementary DNA of a brain calcium channel. Nature. 1991 Apr 4;350(6317):398–402. doi: 10.1038/350398a0. [DOI] [PubMed] [Google Scholar]
- Nawrath H., Wegener J. W. Kinetics and state-dependent effects of verapamil on cardiac L-type calcium channels. Naunyn Schmiedebergs Arch Pharmacol. 1997 Jan;355(1):79–86. doi: 10.1007/pl00004921. [DOI] [PubMed] [Google Scholar]
- Qu Y., Rogers J., Tanada T., Scheuer T., Catterall W. A. Molecular determinants of drug access to the receptor site for antiarrhythmic drugs in the cardiac Na+ channel. Proc Natl Acad Sci U S A. 1995 Dec 5;92(25):11839–11843. doi: 10.1073/pnas.92.25.11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starmer C. F., Grant A. O. Phasic ion channel blockade. A kinetic model and parameter estimation procedure. Mol Pharmacol. 1985 Oct;28(4):348–356. [PubMed] [Google Scholar]
- Timin E. N., Hering S. A method for estimation of drug affinity constants to the open conformational state of calcium channels. Biophys J. 1992 Sep;63(3):808–814. doi: 10.1016/S0006-3495(92)81636-3. [DOI] [PMC free article] [PubMed] [Google Scholar]