Abstract
Adsorption of amphiphilic peptides to the headgroup region of a lipid bilayer is a common mode of protein-membrane interactions. Previous studies have shown that adsorption causes membrane thinning. The degree of the thinning depends on the degree of the lateral expansion caused by the peptide adsorption. If this simple molecular mechanism is correct, the degree of lateral expansion and consequently the membrane thinning should depend on the size of the headgroup relative to the cross section of the hydrocarbon chains. Previously we have established the connection between the alamethicin insertion transition and the membrane thinning effect. In this paper we use oriented circular dichroism to study the effect of varying the size of the headgroup, while maintaining a constant cross section of the lipid chains, on the insertion transition. A simple quantitative prediction agrees very well with the experiment.
Full text
PDF

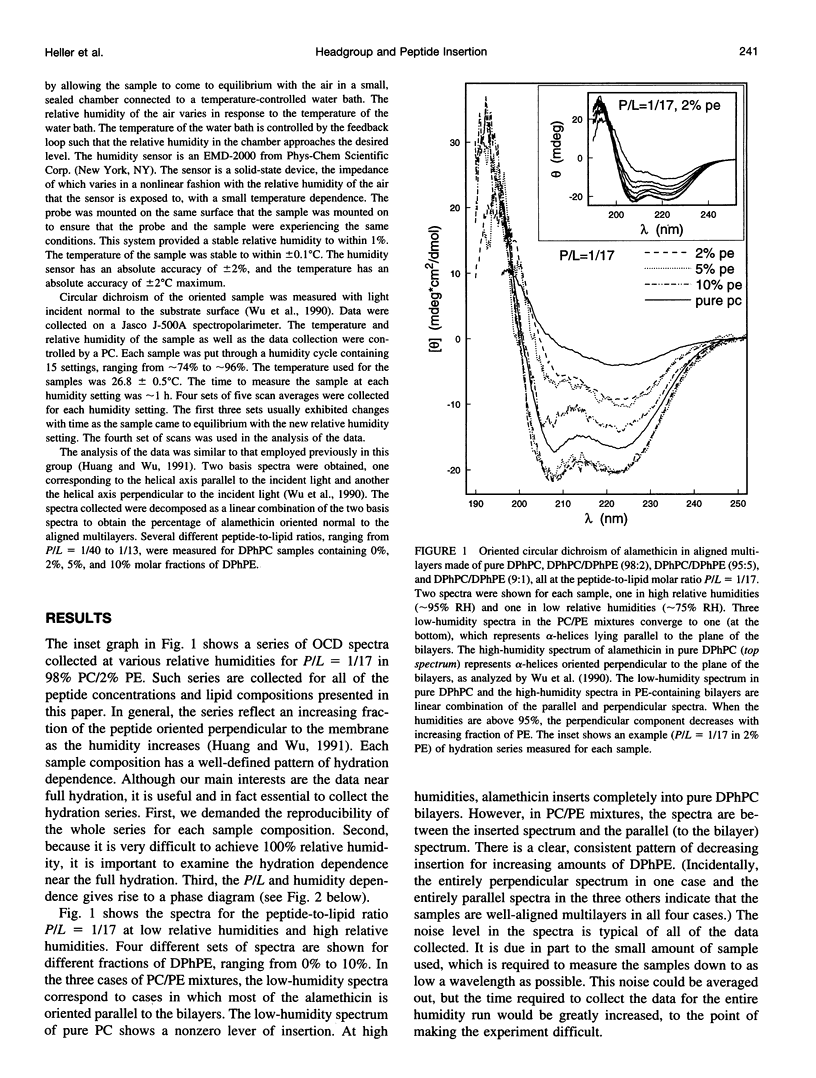


Images in this article
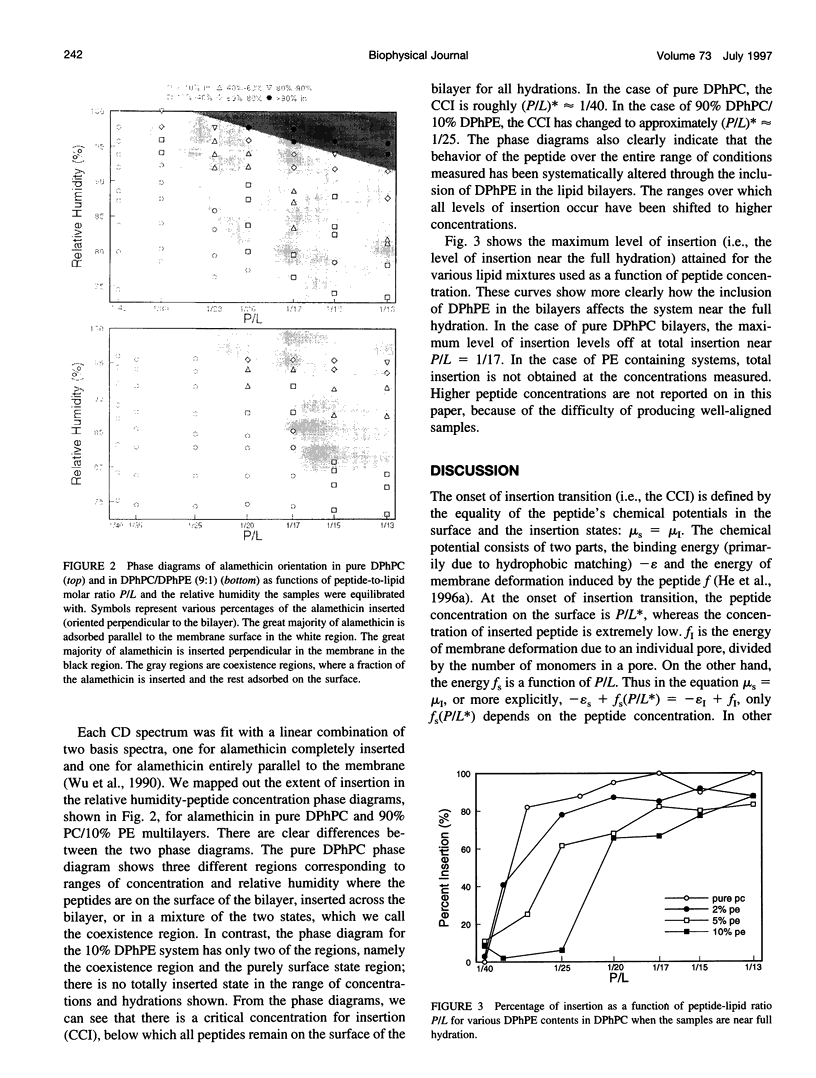
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chothia C. Hydrophobic bonding and accessible surface area in proteins. Nature. 1974 Mar 22;248(446):338–339. doi: 10.1038/248338a0. [DOI] [PubMed] [Google Scholar]
- Epand R. M., Bottega R. Determination of the phase behaviour of phosphatidylethanolamine admixed with other lipids and the effects of calcium chloride: implications for protein kinase C regulation. Biochim Biophys Acta. 1988 Oct 6;944(2):144–154. doi: 10.1016/0005-2736(88)90427-0. [DOI] [PubMed] [Google Scholar]
- Hah J. S., Hui S. W., Jung C. Y. Effects of physical states of phospholipids on the incorporation and cytochalasin B binding activity of human erythrocyte membrane proteins in reconstituted vesicles. Biochemistry. 1983 Sep 27;22(20):4763–4769. doi: 10.1021/bi00289a023. [DOI] [PubMed] [Google Scholar]
- Hauser H., Pascher I., Pearson R. H., Sundell S. Preferred conformation and molecular packing of phosphatidylethanolamine and phosphatidylcholine. Biochim Biophys Acta. 1981 Jun 16;650(1):21–51. doi: 10.1016/0304-4157(81)90007-1. [DOI] [PubMed] [Google Scholar]
- He K., Ludtke S. J., Heller W. T., Huang H. W. Mechanism of alamethicin insertion into lipid bilayers. Biophys J. 1996 Nov;71(5):2669–2679. doi: 10.1016/S0006-3495(96)79458-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K., Ludtke S. J., Huang H. W., Worcester D. L. Antimicrobial peptide pores in membranes detected by neutron in-plane scattering. Biochemistry. 1995 Dec 5;34(48):15614–15618. doi: 10.1021/bi00048a002. [DOI] [PubMed] [Google Scholar]
- He K., Ludtke S. J., Worcester D. L., Huang H. W. Neutron scattering in the plane of membranes: structure of alamethicin pores. Biophys J. 1996 Jun;70(6):2659–2666. doi: 10.1016/S0006-3495(96)79835-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. W., Olah G. A. Uniformly oriented gramicidin channels embedded in thick monodomain lecithin multilayers. Biophys J. 1987 Jun;51(6):989–992. doi: 10.1016/S0006-3495(87)83427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. W., Wu Y. Lipid-alamethicin interactions influence alamethicin orientation. Biophys J. 1991 Nov;60(5):1079–1087. doi: 10.1016/S0006-3495(91)82144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui S. W., Stewart T. P., Yeagle P. L., Albert A. D. Bilayer to non-bilayer transition in mixtures of phosphatidylethanolamine and phosphatidylcholine: implications for membrane properties. Arch Biochem Biophys. 1981 Apr 1;207(2):227–240. doi: 10.1016/0003-9861(81)90029-1. [DOI] [PubMed] [Google Scholar]
- Hultmark D., Steiner H., Rasmuson T., Boman H. G. Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur J Biochem. 1980 May;106(1):7–16. doi: 10.1111/j.1432-1033.1980.tb05991.x. [DOI] [PubMed] [Google Scholar]
- Jen W. C., Jones G. A., Brewer D., Parkinson V. O., Taylor A. The antibacterial activity of alamethicins and zervamicins. J Appl Bacteriol. 1987 Oct;63(4):293–298. doi: 10.1111/j.1365-2672.1987.tb02705.x. [DOI] [PubMed] [Google Scholar]
- Juretić D., Chen H. C., Brown J. H., Morell J. L., Hendler R. W., Westerhoff H. V. Magainin 2 amide and analogues. Antimicrobial activity, membrane depolarization and susceptibility to proteolysis. FEBS Lett. 1989 Jun 5;249(2):219–223. doi: 10.1016/0014-5793(89)80627-1. [DOI] [PubMed] [Google Scholar]
- Keller S. L., Bezrukov S. M., Gruner S. M., Tate M. W., Vodyanoy I., Parsegian V. A. Probability of alamethicin conductance states varies with nonlamellar tendency of bilayer phospholipids. Biophys J. 1993 Jul;65(1):23–27. doi: 10.1016/S0006-3495(93)81040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludtke S. J., He K., Heller W. T., Harroun T. A., Yang L., Huang H. W. Membrane pores induced by magainin. Biochemistry. 1996 Oct 29;35(43):13723–13728. doi: 10.1021/bi9620621. [DOI] [PubMed] [Google Scholar]
- Ludtke S., He K., Huang H. Membrane thinning caused by magainin 2. Biochemistry. 1995 Dec 26;34(51):16764–16769. doi: 10.1021/bi00051a026. [DOI] [PubMed] [Google Scholar]
- Merrifield R. B., Merrifield E. L., Juvvadi P., Andreu D., Boman H. G. Design and synthesis of antimicrobial peptides. Ciba Found Symp. 1994;186:5–26. [PubMed] [Google Scholar]
- Nagle J. F., Wilkinson D. A. Lecithin bilayers. Density measurement and molecular interactions. Biophys J. 1978 Aug;23(2):159–175. doi: 10.1016/S0006-3495(78)85441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. H., Pascher I. The molecular structure of lecithin dihydrate. Nature. 1979 Oct 11;281(5731):499–501. doi: 10.1038/281499a0. [DOI] [PubMed] [Google Scholar]
- Quinn P. J., Chapman D. The dynamics of membrane structure. CRC Crit Rev Biochem. 1980;8(1):1–117. doi: 10.3109/10409238009105466. [DOI] [PubMed] [Google Scholar]
- Steiner H., Andreu D., Merrifield R. B. Binding and action of cecropin and cecropin analogues: antibacterial peptides from insects. Biochim Biophys Acta. 1988 Apr 7;939(2):260–266. doi: 10.1016/0005-2736(88)90069-7. [DOI] [PubMed] [Google Scholar]
- Wiener M. C., Suter R. M., Nagle J. F. Structure of the fully hydrated gel phase of dipalmitoylphosphatidylcholine. Biophys J. 1989 Feb;55(2):315–325. doi: 10.1016/S0006-3495(89)82807-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., He K., Ludtke S. J., Huang H. W. X-ray diffraction study of lipid bilayer membranes interacting with amphiphilic helical peptides: diphytanoyl phosphatidylcholine with alamethicin at low concentrations. Biophys J. 1995 Jun;68(6):2361–2369. doi: 10.1016/S0006-3495(95)80418-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Huang H. W., Olah G. A. Method of oriented circular dichroism. Biophys J. 1990 Apr;57(4):797–806. doi: 10.1016/S0006-3495(90)82599-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]



