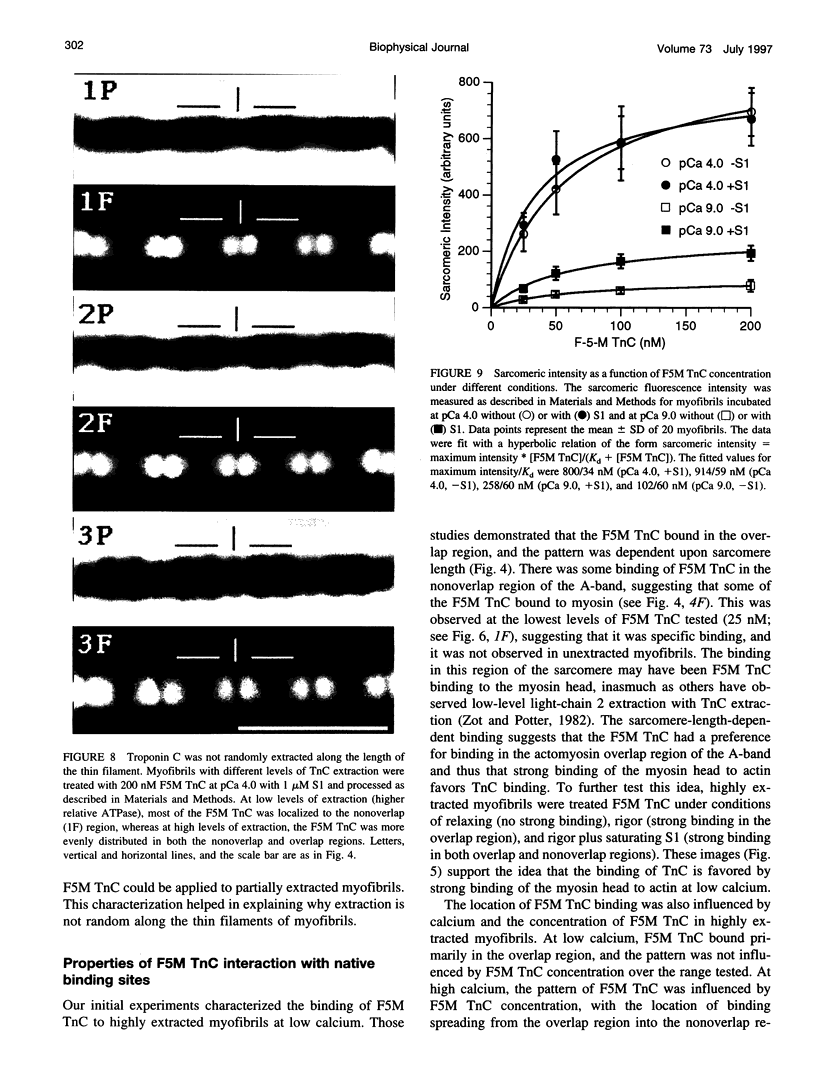
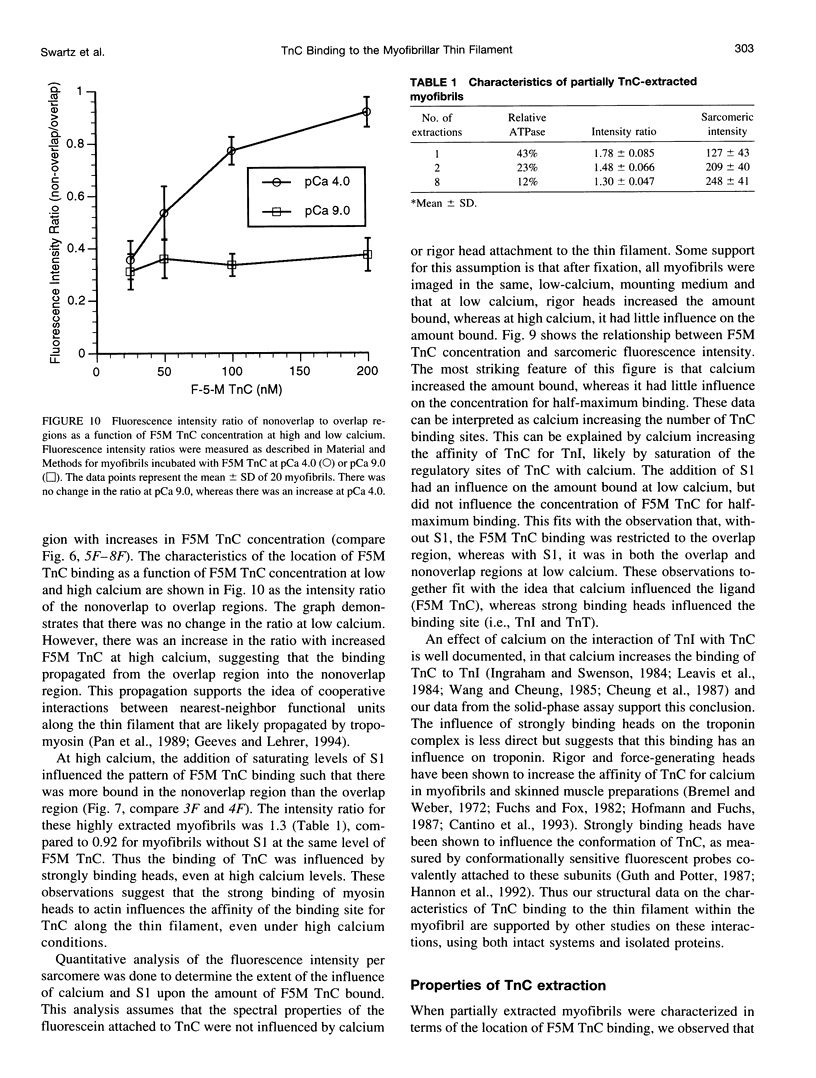
Abstract
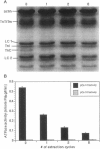
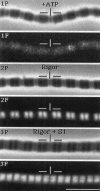
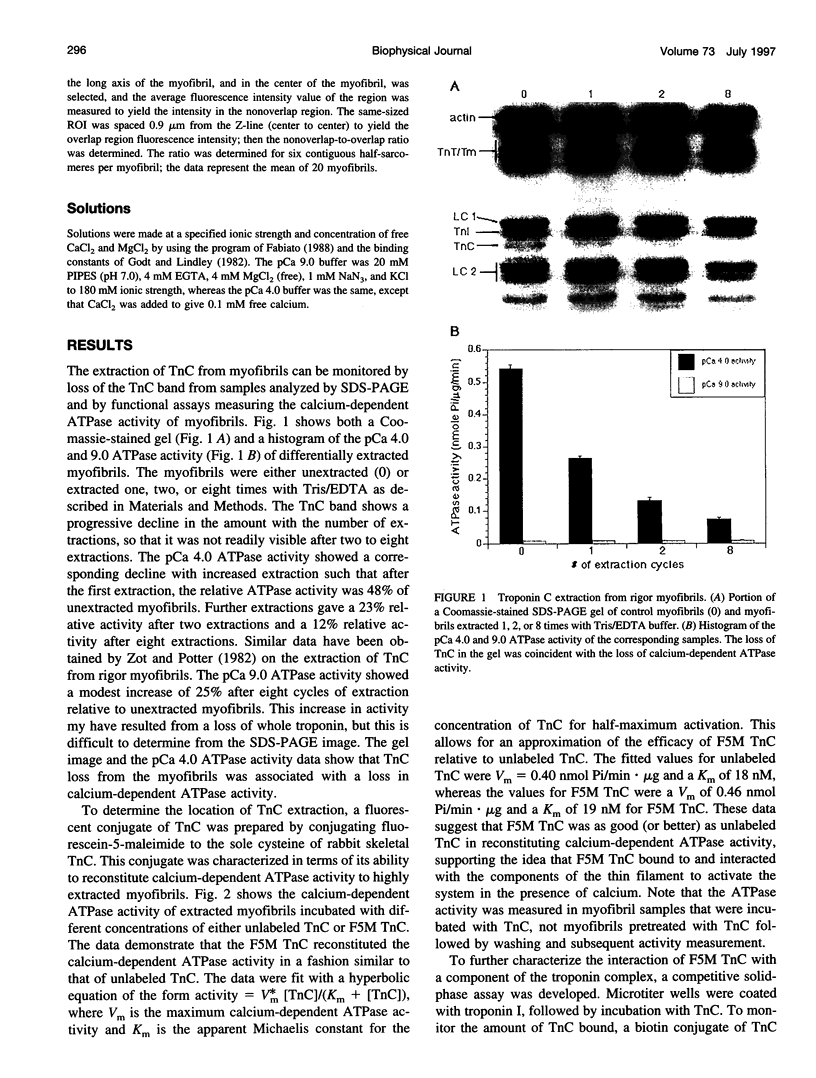
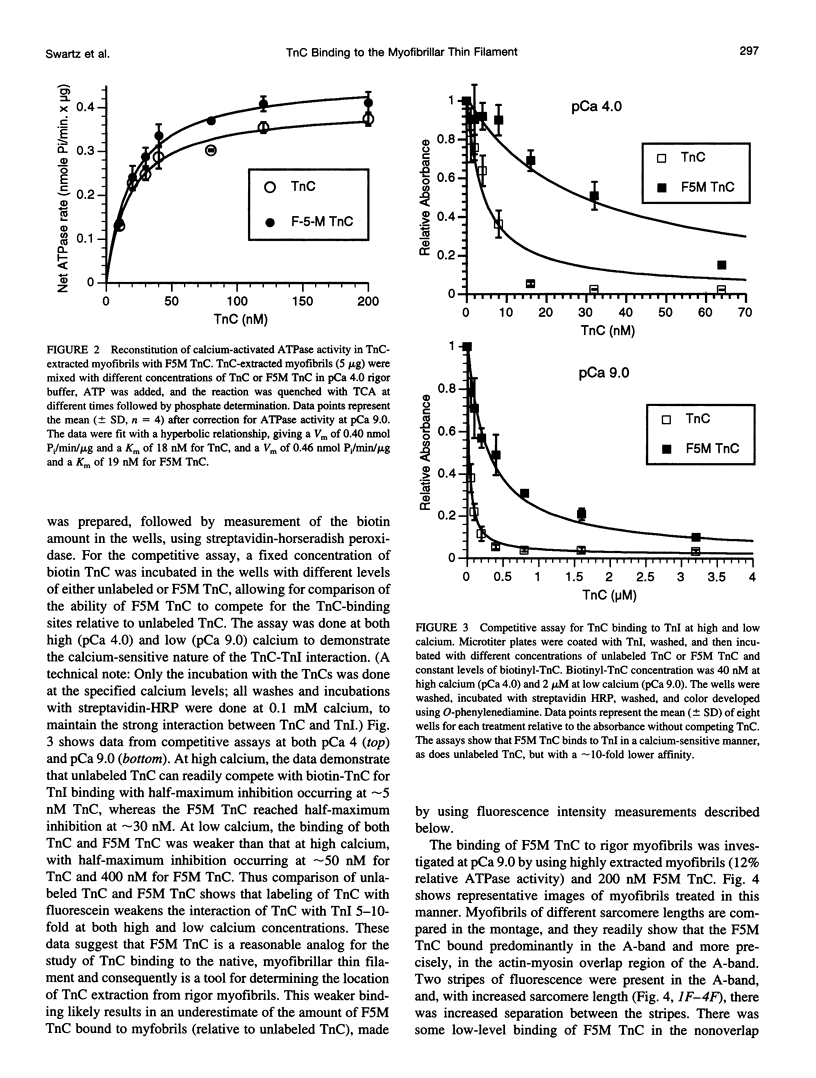
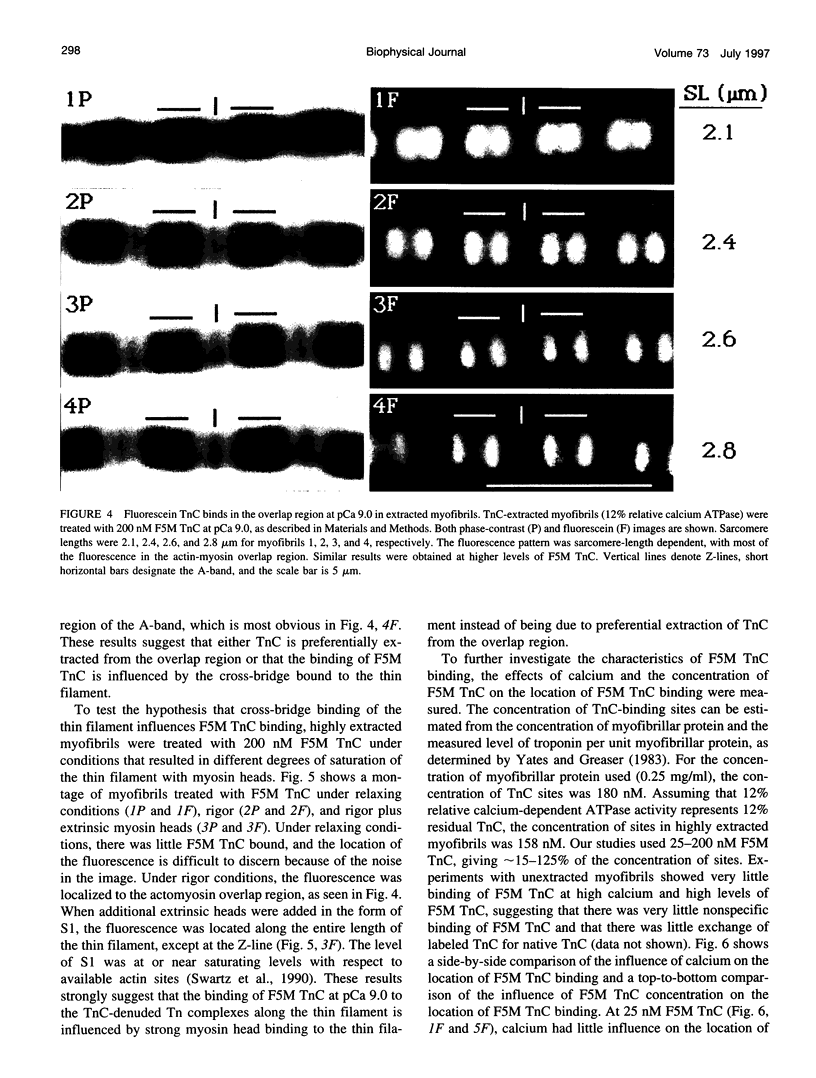
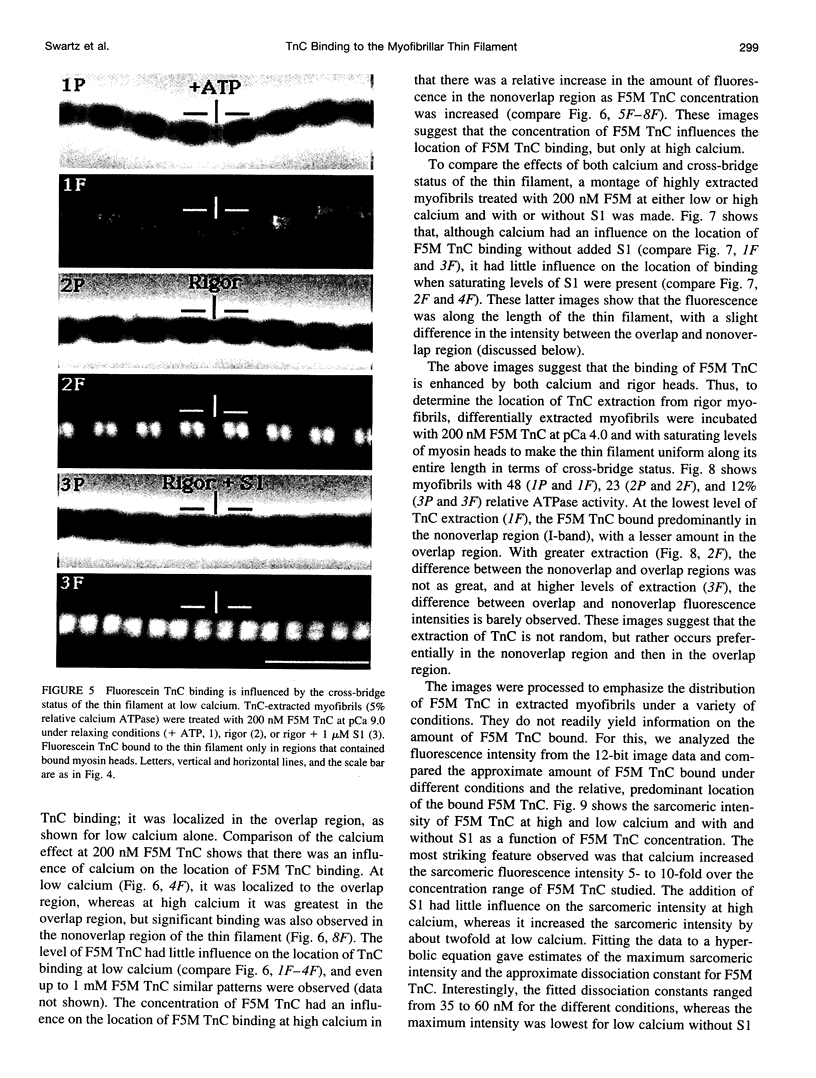
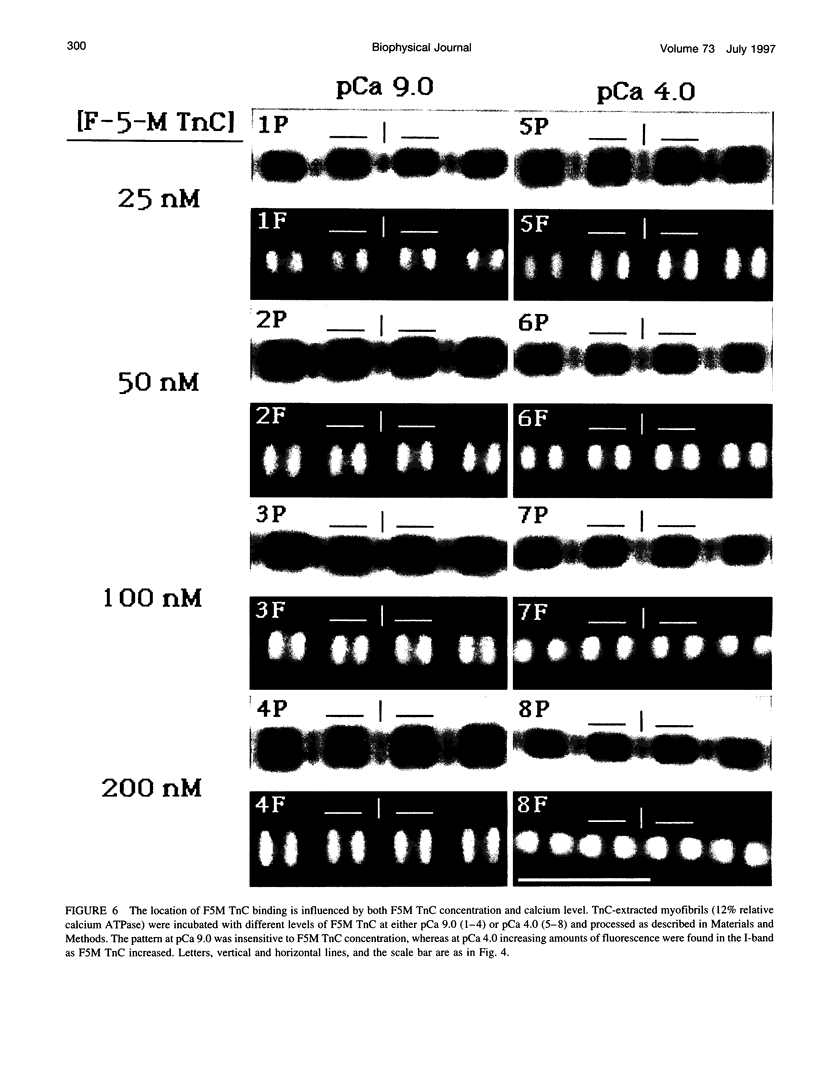
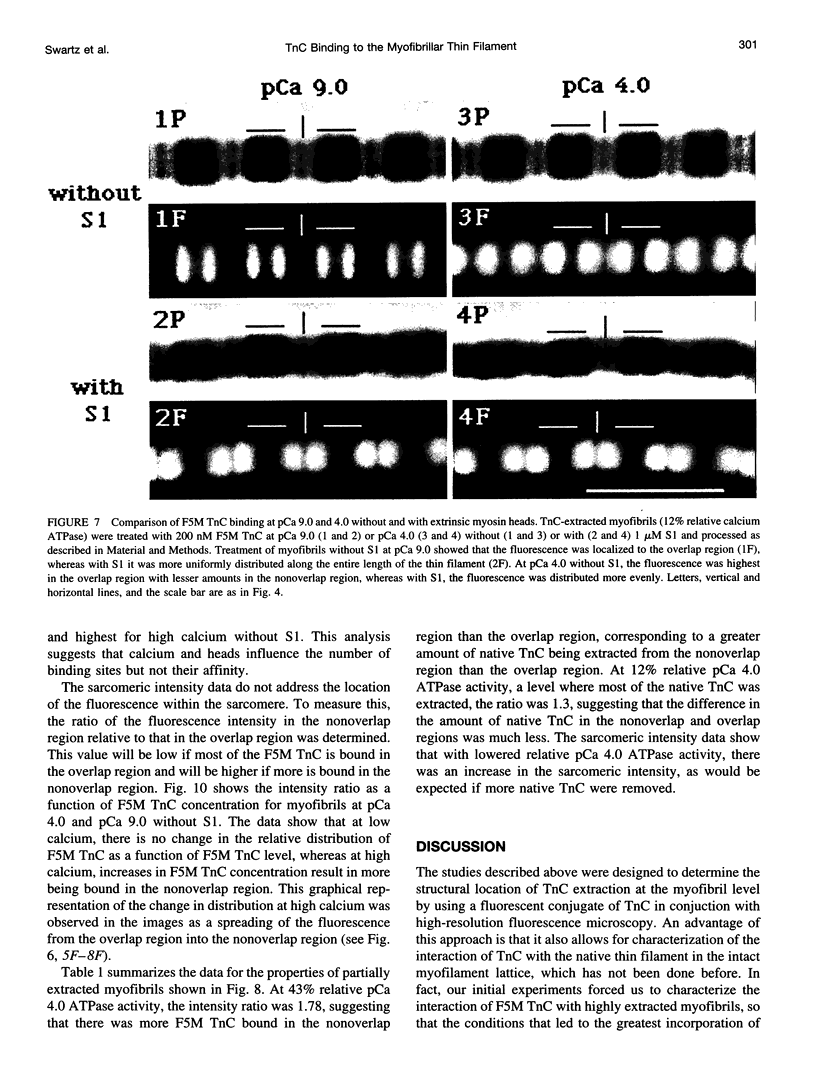
Troponin C (TnC) is the Ca(2+)-sensing subunit of troponin responsible for initiating the cascade of events resulting in contraction of striated muscle. This protein can be readily extracted from myofibrils with low-ionic-strength EDTA-containing buffers. The properties of TnC extraction have not been characterized at the structural level, nor have the interactions of TnC with the native myofibrillar thin filament been studied. To address these issues, fluorescein-labeled TnC, in conjunction with high-resolution digital fluorescence microscopy, was used to characterize TnC binding to myofibrils and to determine the randomness of TnC extraction. Fluorescein-5-maleimide TnC (F5M TnC) retained biological activity, as evidenced by reconstitution of Ca(2+)-dependent ATPase activity in extracted myofibrils and binding to TnI in a Ca(2+)-sensitive manner. The binding of F5M TnC to highly extracted myofibrils at low Ca2+ was restricted to the overlap region under rigor conditions, and the location of binding was not influenced by F5M TnC concentration. The addition of myosin subfragment 1 to occupy all actin sites resulted in F5M TnC being bound in both the overlap and nonoverlap regions. However, very little F5M TnC was bound to myofibrils under relaxing conditions. These results suggest that strong binding of myosin heads enhances TnC binding. At high Ca2+, the pattern of F5M TnC binding was concentration dependent: binding was restricted to the overlap region at low F5M TnC concentration, whereas the binding propagated into the nonoverlap region at higher levels. Analysis of fluorescence intensity showed the greatest binding of F5M TnC at high Ca2+ with S1, and these conditions were used to characterize partially TnC-extracted myofibrils. Comparison of partially extracted myofibrils showed that low levels of extraction were associated with greater F5M TnC being bound in the nonoverlap region than in the overlap region relative to higher levels of extraction. These results show that TnC extraction is not random along the length of the thin filament, but occurs more readily in the nonoverlap region. This observation, in conjunction with the influence of rigor heads on the pattern of F5M TnC binding, suggests that strong myosin binding to actin stabilizes TnC binding at low Ca2+.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babu A., Rao V. G., Su H., Gulati J. Critical minimum length of the central helix in troponin C for the Ca2+ switch in muscular contraction. J Biol Chem. 1993 Sep 15;268(26):19232–19238. [PubMed] [Google Scholar]
- Babu A., Scordilis S. P., Sonnenblick E. H., Gulati J. The control of myocardial contraction with skeletal fast muscle troponin C. J Biol Chem. 1987 Apr 25;262(12):5815–5822. [PubMed] [Google Scholar]
- Babu A., Sonnenblick E., Gulati J. Molecular basis for the influence of muscle length on myocardial performance. Science. 1988 Apr 1;240(4848):74–76. doi: 10.1126/science.3353709. [DOI] [PubMed] [Google Scholar]
- Brandt P. W., Diamond M. S., Rutchik J. S., Schachat F. H. Co-operative interactions between troponin-tropomyosin units extend the length of the thin filament in skeletal muscle. J Mol Biol. 1987 Jun 20;195(4):885–896. doi: 10.1016/0022-2836(87)90492-x. [DOI] [PubMed] [Google Scholar]
- Bremel R. D., Weber A. Cooperation within actin filament in vertebrate skeletal muscle. Nat New Biol. 1972 Jul 26;238(82):97–101. doi: 10.1038/newbio238097a0. [DOI] [PubMed] [Google Scholar]
- Cantino M. E., Allen T. S., Gordon A. M. Subsarcomeric distribution of calcium in demembranated fibers of rabbit psoas muscle. Biophys J. 1993 Jan;64(1):211–222. doi: 10.1016/S0006-3495(93)81358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter S. G., Karl D. W. Inorganic phosphate assay with malachite green: an improvement and evaluation. J Biochem Biophys Methods. 1982 Dec;7(1):7–13. doi: 10.1016/0165-022x(82)90031-8. [DOI] [PubMed] [Google Scholar]
- Cheung H. C., Wang C. K., Malik N. A. Interactions of troponin subunits: free energy of binary and ternary complexes. Biochemistry. 1987 Sep 8;26(18):5904–5907. doi: 10.1021/bi00392a049. [DOI] [PubMed] [Google Scholar]
- Cox J. A., Comte M., Stein E. A. Calmodulin-free skeletal-muscle troponin C prepared in the absence of urea. Biochem J. 1981 Apr 1;195(1):205–211. doi: 10.1042/bj1950205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Fritz J. D., Swartz D. R., Greaser M. L. Factors affecting polyacrylamide gel electrophoresis and electroblotting of high-molecular-weight myofibrillar proteins. Anal Biochem. 1989 Aug 1;180(2):205–210. doi: 10.1016/0003-2697(89)90116-4. [DOI] [PubMed] [Google Scholar]
- Fuchs F., Fox C. Parallel measurements of bound calcium and force in glycerinated rabbit psoas muscle fibers. Biochim Biophys Acta. 1982 Jan 20;679(1):110–115. doi: 10.1016/0005-2728(82)90261-4. [DOI] [PubMed] [Google Scholar]
- GORNALL A. G., BARDAWILL C. J., DAVID M. M. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949 Feb;177(2):751–766. [PubMed] [Google Scholar]
- Geeves M. A., Lehrer S. S. Dynamics of the muscle thin filament regulatory switch: the size of the cooperative unit. Biophys J. 1994 Jul;67(1):273–282. doi: 10.1016/S0006-3495(94)80478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt R. E., Lindley B. D. Influence of temperature upon contractile activation and isometric force production in mechanically skinned muscle fibers of the frog. J Gen Physiol. 1982 Aug;80(2):279–297. doi: 10.1085/jgp.80.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabarek Z., Tao T., Gergely J. Molecular mechanism of troponin-C function. J Muscle Res Cell Motil. 1992 Aug;13(4):383–393. doi: 10.1007/BF01738034. [DOI] [PubMed] [Google Scholar]
- Greaser M. L., Gergely J. Reconstitution of troponin activity from three protein components. J Biol Chem. 1971 Jul 10;246(13):4226–4233. [PubMed] [Google Scholar]
- Güth K., Potter J. D. Effect of rigor and cycling cross-bridges on the structure of troponin C and on the Ca2+ affinity of the Ca2+-specific regulatory sites in skinned rabbit psoas fibers. J Biol Chem. 1987 Oct 5;262(28):13627–13635. [PubMed] [Google Scholar]
- Hannon J. D., Martyn D. A., Gordon A. M. Effects of cycling and rigor crossbridges on the conformation of cardiac troponin C. Circ Res. 1992 Oct;71(4):984–991. doi: 10.1161/01.res.71.4.984. [DOI] [PubMed] [Google Scholar]
- Hannon J. D., Martyn D. A., Gordon A. M. Effects of cycling and rigor crossbridges on the conformation of cardiac troponin C. Circ Res. 1992 Oct;71(4):984–991. doi: 10.1161/01.res.71.4.984. [DOI] [PubMed] [Google Scholar]
- Herzberg O., James M. N. Refined crystal structure of troponin C from turkey skeletal muscle at 2.0 A resolution. J Mol Biol. 1988 Oct 5;203(3):761–779. doi: 10.1016/0022-2836(88)90208-2. [DOI] [PubMed] [Google Scholar]
- Hill H. D., Straka J. G. Protein determination using bicinchoninic acid in the presence of sulfhydryl reagents. Anal Biochem. 1988 Apr;170(1):203–208. doi: 10.1016/0003-2697(88)90109-1. [DOI] [PubMed] [Google Scholar]
- Hofmann P. A., Fuchs F. Evidence for a force-dependent component of calcium binding to cardiac troponin C. Am J Physiol. 1987 Oct;253(4 Pt 1):C541–C546. doi: 10.1152/ajpcell.1987.253.4.C541. [DOI] [PubMed] [Google Scholar]
- Ingraham R. H., Swenson C. A. Binary interactions of troponin subunits. J Biol Chem. 1984 Aug 10;259(15):9544–9548. [PubMed] [Google Scholar]
- Kerrick W. G., Zot H. G., Hoar P. E., Potter J. D. Evidence that the Sr2+ activation properties of cardiac troponin C are altered when substituted into skinned skeletal muscle fibers. J Biol Chem. 1985 Dec 15;260(29):15687–15693. [PubMed] [Google Scholar]
- Leavis P. C., Gergely J. Thin filament proteins and thin filament-linked regulation of vertebrate muscle contraction. CRC Crit Rev Biochem. 1984;16(3):235–305. doi: 10.3109/10409238409108717. [DOI] [PubMed] [Google Scholar]
- Leavis P. C., Gowell E., Tao T. Fluorescence lifetime and acrylamide quenching studies of the interactions between troponin subunits. Biochemistry. 1984 Aug 28;23(18):4156–4161. doi: 10.1021/bi00313a023. [DOI] [PubMed] [Google Scholar]
- Moss R. L., Giulian G. G., Greaser M. L. The effects of partial extraction of TnC upon the tension-pCa relationship in rabbit skinned skeletal muscle fibers. J Gen Physiol. 1985 Oct;86(4):585–600. doi: 10.1085/jgp.86.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R. L., Lauer M. R., Giulian G. G., Greaser M. L. Altered Ca2+ dependence of tension development in skinned skeletal muscle fibers following modification of troponin by partial substitution with cardiac troponin C. J Biol Chem. 1986 May 5;261(13):6096–6099. [PubMed] [Google Scholar]
- Moss R. L., Nwoye L. O., Greaser M. L. Substitution of cardiac troponin C into rabbit muscle does not alter the length dependence of Ca2+ sensitivity of tension. J Physiol. 1991;440:273–289. doi: 10.1113/jphysiol.1991.sp018708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B. S., Gordon A. M., Luo Z. X. Removal of tropomyosin overlap modifies cooperative binding of myosin S-1 to reconstituted thin filaments of rabbit striated muscle. J Biol Chem. 1989 May 25;264(15):8495–8498. [PubMed] [Google Scholar]
- Potter J. D., Gergely J. The calcium and magnesium binding sites on troponin and their role in the regulation of myofibrillar adenosine triphosphatase. J Biol Chem. 1975 Jun 25;250(12):4628–4633. [PubMed] [Google Scholar]
- Potter J. D. Preparation of troponin and its subunits. Methods Enzymol. 1982;85(Pt B):241–263. doi: 10.1016/0076-6879(82)85024-6. [DOI] [PubMed] [Google Scholar]
- Satyshur K. A., Pyzalska D., Greaser M., Rao S. T., Sundaralingam M. Structure of chicken skeletal muscle troponin C at 1.78 A resolution. Acta Crystallogr D Biol Crystallogr. 1994 Jan 1;50(Pt 1):40–49. doi: 10.1107/S090744499300798X. [DOI] [PubMed] [Google Scholar]
- Satyshur K. A., Rao S. T., Pyzalska D., Drendel W., Greaser M., Sundaralingam M. Refined structure of chicken skeletal muscle troponin C in the two-calcium state at 2-A resolution. J Biol Chem. 1988 Feb 5;263(4):1628–1647. [PubMed] [Google Scholar]
- Slupsky C. M., Sykes B. D. NMR solution structure of calcium-saturated skeletal muscle troponin C. Biochemistry. 1995 Dec 12;34(49):15953–15964. doi: 10.1021/bi00049a010. [DOI] [PubMed] [Google Scholar]
- Sorenson M. M., da Silva A. C., Gouveia C. S., Sousa V. P., Oshima W., Ferro J. A., Reinach F. C. Concerted action of the high affinity calcium binding sites in skeletal muscle troponin C. J Biol Chem. 1995 Apr 28;270(17):9770–9777. doi: 10.1074/jbc.270.17.9770. [DOI] [PubMed] [Google Scholar]
- Swartz D. R., Greaser M. L., Marsh B. B. Regulation of binding of subfragment 1 in isolated rigor myofibrils. J Cell Biol. 1990 Dec;111(6 Pt 2):2989–3001. doi: 10.1083/jcb.111.6.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz D. R., Moss R. L., Greaser M. L. Calcium alone does not fully activate the thin filament for S1 binding to rigor myofibrils. Biophys J. 1996 Oct;71(4):1891–1904. doi: 10.1016/S0006-3495(96)79388-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz D. R., Moss R. L. Influence of a strong-binding myosin analogue on calcium-sensitive mechanical properties of skinned skeletal muscle fibers. J Biol Chem. 1992 Oct 5;267(28):20497–20506. [PubMed] [Google Scholar]
- Sweeney H. L., Brito R. M., Rosevear P. R., Putkey J. A. The low-affinity Ca2(+)-binding sites in cardiac/slow skeletal muscle troponin C perform distinct functions: site I alone cannot trigger contraction. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9538–9542. doi: 10.1073/pnas.87.24.9538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczesna D., Guzman G., Miller T., Zhao J., Farokhi K., Ellemberger H., Potter J. D. The role of the four Ca2+ binding sites of troponin C in the regulation of skeletal muscle contraction. J Biol Chem. 1996 Apr 5;271(14):8381–8386. doi: 10.1074/jbc.271.14.8381. [DOI] [PubMed] [Google Scholar]
- Wagner P. D., Weeds A. G. Studies on the role of myosin alkali light chains. Recombination and hybridization of light chains and heavy chains in subfragment-1 preparations. J Mol Biol. 1977 Jan 25;109(3):455–470. doi: 10.1016/s0022-2836(77)80023-5. [DOI] [PubMed] [Google Scholar]
- Wang C. K., Cheung H. C. Energetics of the binding of calcium and troponin I to troponin C from rabbit skeletal muscle. Biophys J. 1985 Nov;48(5):727–739. doi: 10.1016/S0006-3495(85)83831-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeds A. G., Pope B. Studies on the chymotryptic digestion of myosin. Effects of divalent cations on proteolytic susceptibility. J Mol Biol. 1977 Apr;111(2):129–157. doi: 10.1016/s0022-2836(77)80119-8. [DOI] [PubMed] [Google Scholar]
- Yates L. D., Coby R. L., Luo Z., Gordon A. M. Filament overlap affects TnC extraction from skinned muscle fibres. J Muscle Res Cell Motil. 1993 Aug;14(4):392–400. doi: 10.1007/BF00121290. [DOI] [PubMed] [Google Scholar]
- Yates L. D., Greaser M. L. Quantitative determination of myosin and actin in rabbit skeletal muscle. J Mol Biol. 1983 Jul 25;168(1):123–141. doi: 10.1016/s0022-2836(83)80326-x. [DOI] [PubMed] [Google Scholar]
- Zot A. S., Potter J. D. Reciprocal coupling between troponin C and myosin crossbridge attachment. Biochemistry. 1989 Aug 8;28(16):6751–6756. doi: 10.1021/bi00442a031. [DOI] [PubMed] [Google Scholar]
- Zot H. G., Güth K., Potter J. D. Fast skeletal muscle skinned fibers and myofibrils reconstituted with N-terminal fluorescent analogues of troponin C. J Biol Chem. 1986 Dec 5;261(34):15883–15890. [PubMed] [Google Scholar]
- Zot H. G., Potter J. D. A structural role for the Ca2+-Mg2+ sites on troponin C in the regulation of muscle contraction. Preparation and properties of troponin C depleted myofibrils. J Biol Chem. 1982 Jul 10;257(13):7678–7683. [PubMed] [Google Scholar]