Abstract
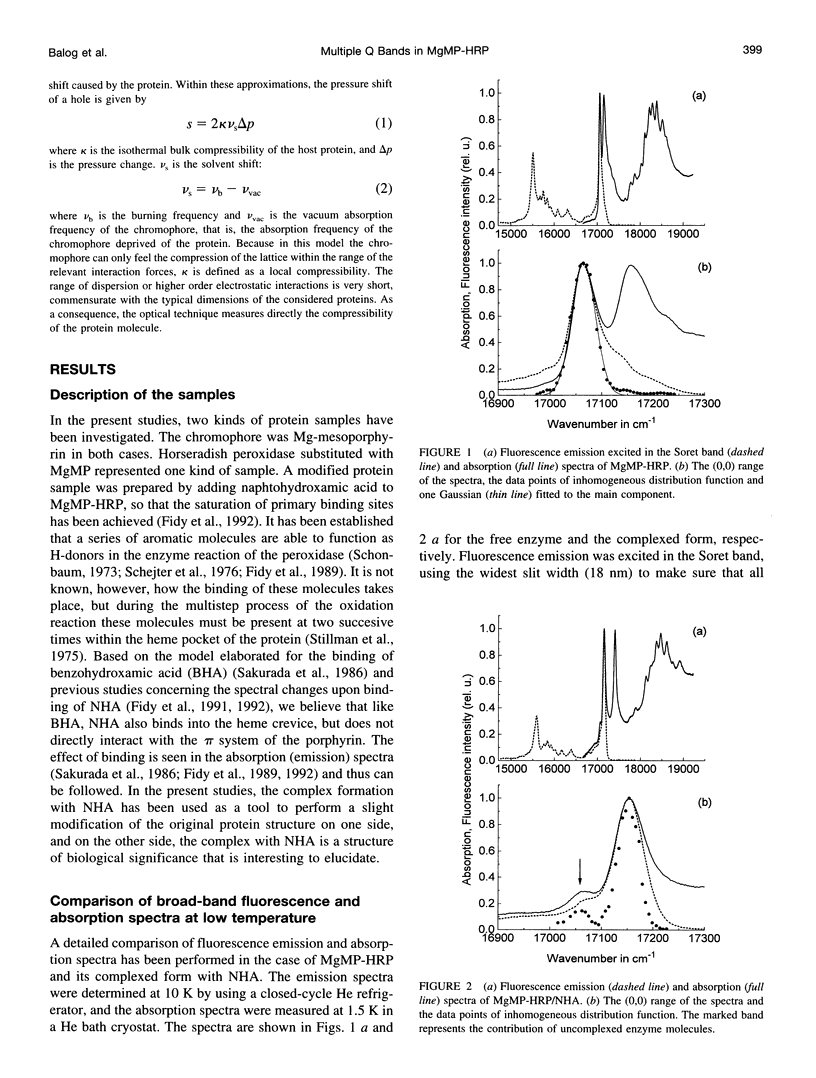
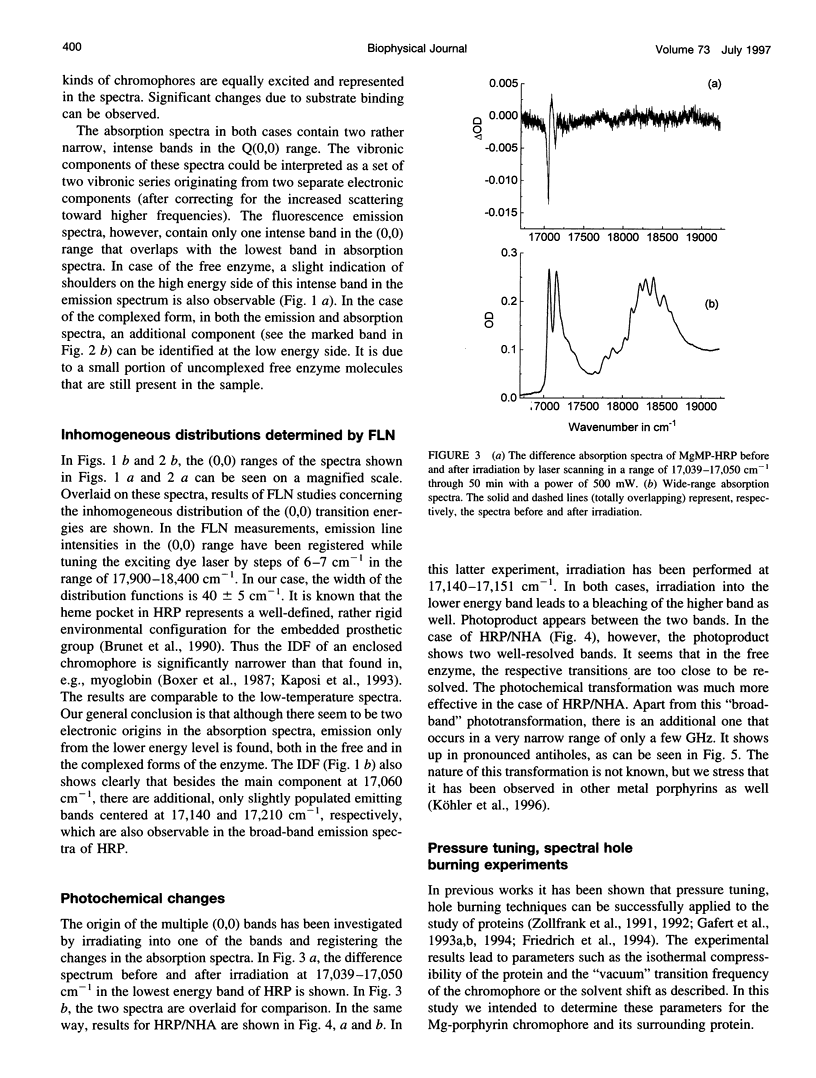
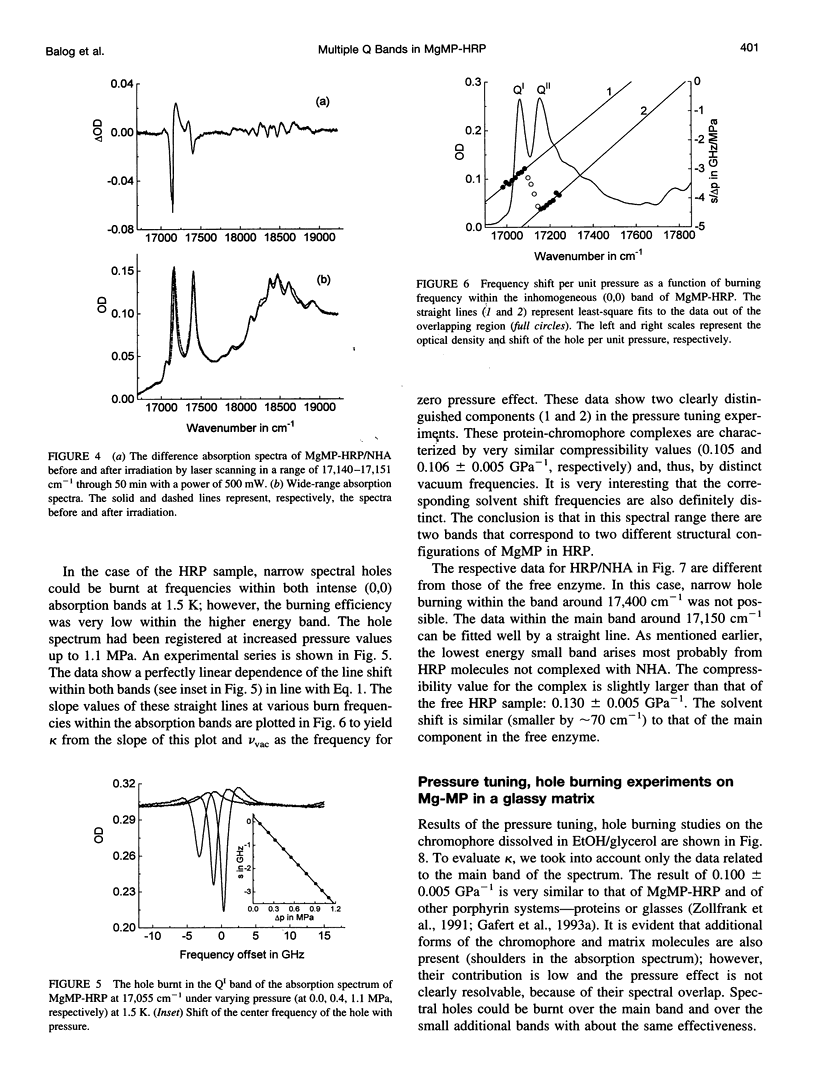
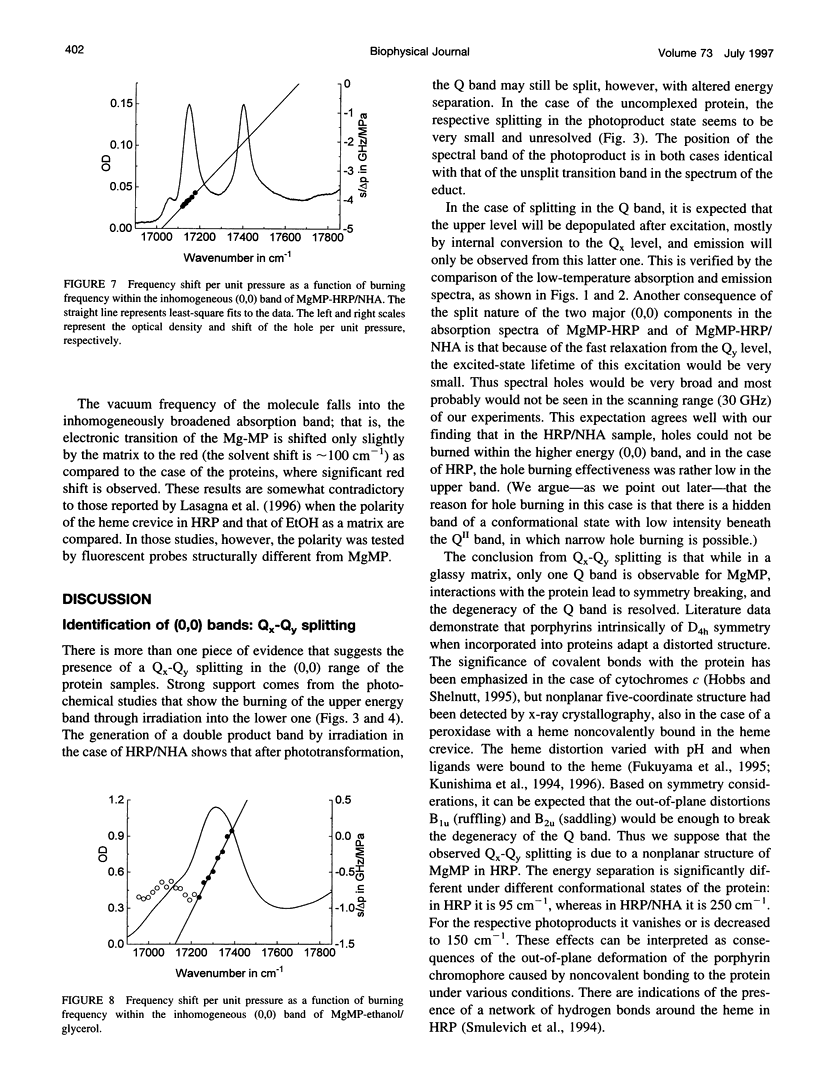
Mg-mesoporphyrin horseradish peroxidase (MgMP-HRP) and MgMP-HRP complexed with naphtohydroxamic acid (NHA) have been studied by fluorescence line narrowing (FLN) and pressure tuning spectral hole burning (SHB) techniques. In each sample, the low temperature absorption spectra show more than one transition in the origin range of the Q band. Comparisons with broad-band fluorescence spectra and FLN studies suggest that the multiple band feature originates from the presence of different configurations of the metal-porphyrin that are subject to Qx-Qy splitting within the protein cavity. This suggestion is supported by pressure tuning SHB studies. In the uncomplexed as well as in the NHA-complexed form of MgMP-HRP, irradiation in the Q band produces photoproduct bands, which has been attributed to a species with smaller Qx-Qy splitting. In an amorphous matrix, on the other hand, only one form of MgMP could be found, and no splitting could be observed. The binding of NHA does not significantly alter the bulk parameters of the protein matrix, but it reduces the structural variety in the configuration of MgMP to a single form with a more distorted structure and thus with an enlarged Qx-Qy splitting.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunet J. E., Jullian C., Jameson D. M. Oxygen diffusion through horseradish peroxidase. Photochem Photobiol. 1990 Apr;51(4):487–489. doi: 10.1111/j.1751-1097.1990.tb01742.x. [DOI] [PubMed] [Google Scholar]
- Champion P. M., Collins D. W., Fitchen D. B. Resonance Raman spectra of heme proteins at low temperature. J Am Chem Soc. 1976 Oct 27;98(22):7114–7115. doi: 10.1021/ja00438a081. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J., Michel H. The Photosynthetic Reaction Center from the Purple Bacterium Rhodopseudomonas viridis. Science. 1989 Sep 29;245(4925):1463–1473. doi: 10.1126/science.245.4925.1463. [DOI] [PubMed] [Google Scholar]
- Fidy J., Paul K. G., Vanderkooi J. M. Differences in the binding of aromatic substrates to horseradish peroxidase revealed by fluorescence line narrowing. Biochemistry. 1989 Sep 19;28(19):7531–7541. doi: 10.1021/bi00445a006. [DOI] [PubMed] [Google Scholar]
- Fidy J., Vanderkooi J. M., Zollfrank J., Friedrich J. Softening of the packing density of horseradish peroxidase by a H-donor bound near the heme pocket. Biophys J. 1992 Dec;63(6):1605–1612. doi: 10.1016/S0006-3495(92)81749-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J. M., Rousseau D. L., Adar F. Excited state lifetimes in cytochromes measured from Raman scattering data: evidence for iron-porphyrin interactions. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2607–2611. doi: 10.1073/pnas.74.7.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich J., Gafert J., Zollfrank J., Vanderkooi J., Fidy J. Spectral hole burning and selection of conformational substates in chromoproteins. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):1029–1033. doi: 10.1073/pnas.91.3.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyama K., Kunishima N., Amada F., Kubota T., Matsubara H. Crystal structures of cyanide- and triiodide-bound forms of Arthromyces ramosus peroxidase at different pH values. Perturbations of active site residues and their implication in enzyme catalysis. J Biol Chem. 1995 Sep 15;270(37):21884–21892. doi: 10.1074/jbc.270.37.21884. [DOI] [PubMed] [Google Scholar]
- Gavish B., Gratton E., Hardy C. J. Adiabatic compressibility of globular proteins. Proc Natl Acad Sci U S A. 1983 Feb;80(3):750–754. doi: 10.1073/pnas.80.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs J. D., Shelnutt J. A. Conserved nonplanar heme distortions in cytochromes c. J Protein Chem. 1995 Jan;14(1):19–25. doi: 10.1007/BF01902840. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Tamura M., Hayashi K., Hori H., Morimoto H. Electron paramagnetic resonance and optical absorption spectrum of the pentacoordinated ferrihemoproteins. J Biol Chem. 1980 Mar 25;255(6):2239–2242. [PubMed] [Google Scholar]
- Kunishima N., Amada F., Fukuyama K., Kawamoto M., Matsunaga T., Matsubara H. Pentacoordination of the heme iron of Arthromyces ramosus peroxidase shown by a 1.8 A resolution crystallographic study at pH 4.5. FEBS Lett. 1996 Jan 15;378(3):291–294. doi: 10.1016/0014-5793(95)01458-6. [DOI] [PubMed] [Google Scholar]
- Kunishima N., Fukuyama K., Matsubara H., Hatanaka H., Shibano Y., Amachi T. Crystal structure of the fungal peroxidase from Arthromyces ramosus at 1.9 A resolution. Structural comparisons with the lignin and cytochrome c peroxidases. J Mol Biol. 1994 Jan 7;235(1):331–344. doi: 10.1016/s0022-2836(05)80037-3. [DOI] [PubMed] [Google Scholar]
- Köhler M., Gafert J., Friedrich J., Vanderkooi J. M., Laberge M. Stark effect experiments in cytochrome c-type proteins: structural hierarchies. Biophys J. 1996 Jul;71(1):77–85. doi: 10.1016/S0006-3495(96)79237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladner R. C., Heidner E. J., Perutz M. F. The structure of horse methaemoglobin at 2-0 A resolution. J Mol Biol. 1977 Aug 15;114(3):385–414. doi: 10.1016/0022-2836(77)90256-x. [DOI] [PubMed] [Google Scholar]
- Lasagna M., Vargas V., Jameson D. M., Brunet J. E. Spectral properties of environmentally sensitive probes associated with horseradish peroxidase. Biochemistry. 1996 Jan 23;35(3):973–979. doi: 10.1021/bi951983t. [DOI] [PubMed] [Google Scholar]
- Sakurada J., Takahashi S., Hosoya T. Nuclear magnetic resonance studies on the spatial relationship of aromatic donor molecules to the heme iron of horseradish peroxidase. J Biol Chem. 1986 Jul 25;261(21):9657–9662. [PubMed] [Google Scholar]
- Schejter A., Lanir A., Epstein N. Binding of hydrogen donors to horseradish peroxidase: a spectroscopic study. Arch Biochem Biophys. 1976 May;174(1):36–44. doi: 10.1016/0003-9861(76)90321-0. [DOI] [PubMed] [Google Scholar]
- Schonbaum G. R. New complexes of peroxidases with hydroxamic acids, hydrazides, and amides. J Biol Chem. 1973 Jan 25;248(2):502–511. [PubMed] [Google Scholar]
- Smulevich G., Paoli M., Burke J. F., Sanders S. A., Thorneley R. N., Smith A. T. Characterization of recombinant horseradish peroxidase C and three site-directed mutants, F41V, F41W, and R38K, by resonance Raman spectroscopy. Biochemistry. 1994 Jun 14;33(23):7398–7407. doi: 10.1021/bi00189a046. [DOI] [PubMed] [Google Scholar]
- Stillman J. S., Stillman M. J., Dunford H. B. Photochemical reactions of horseradish peroxidase compounds I and II at room temperature and 13 degrees K. Biochemistry. 1975 Jul 15;14(14):3183–3188. doi: 10.1021/bi00685a023. [DOI] [PubMed] [Google Scholar]
- TEALE F. W. Cleavage of the haem-protein link by acid methylethylketone. Biochim Biophys Acta. 1959 Oct;35:543–543. doi: 10.1016/0006-3002(59)90407-x. [DOI] [PubMed] [Google Scholar]
- Takano T. Structure of myoglobin refined at 2-0 A resolution. I. Crystallographic refinement of metmyoglobin from sperm whale. J Mol Biol. 1977 Mar 5;110(3):537–568. doi: 10.1016/s0022-2836(77)80111-3. [DOI] [PubMed] [Google Scholar]
- Takano T. Structure of myoglobin refined at 2-0 A resolution. II. Structure of deoxymyoglobin from sperm whale. J Mol Biol. 1977 Mar 5;110(3):569–584. doi: 10.1016/s0022-2836(77)80112-5. [DOI] [PubMed] [Google Scholar]
- Teraoka J., Kitagawa T. Structural implication of the heme-linked ionization of horseradish peroxidase probed by the Fe-histidine stretching Raman line. J Biol Chem. 1981 Apr 25;256(8):3969–3977. [PubMed] [Google Scholar]
- Tronrud D. E., Schmid M. F., Matthews B. W. Structure and X-ray amino acid sequence of a bacteriochlorophyll A protein from Prosthecochloris aestuarii refined at 1.9 A resolution. J Mol Biol. 1986 Apr 5;188(3):443–454. doi: 10.1016/0022-2836(86)90167-1. [DOI] [PubMed] [Google Scholar]
- Wagner G. C., Kassner J. Spectroscopic properties of low-spin ferrous heme complexes and hemeproteins at 77degrees K. Biochem Biophys Res Commun. 1975 Mar 17;63(2):385–391. doi: 10.1016/0006-291x(75)90700-7. [DOI] [PubMed] [Google Scholar]
- Zollfrank J., Friedrich J., Parak F. Spectral hole burning study of protoporphyrin IX substituted myoglobin. Biophys J. 1992 Mar;61(3):716–724. doi: 10.1016/S0006-3495(92)81876-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


