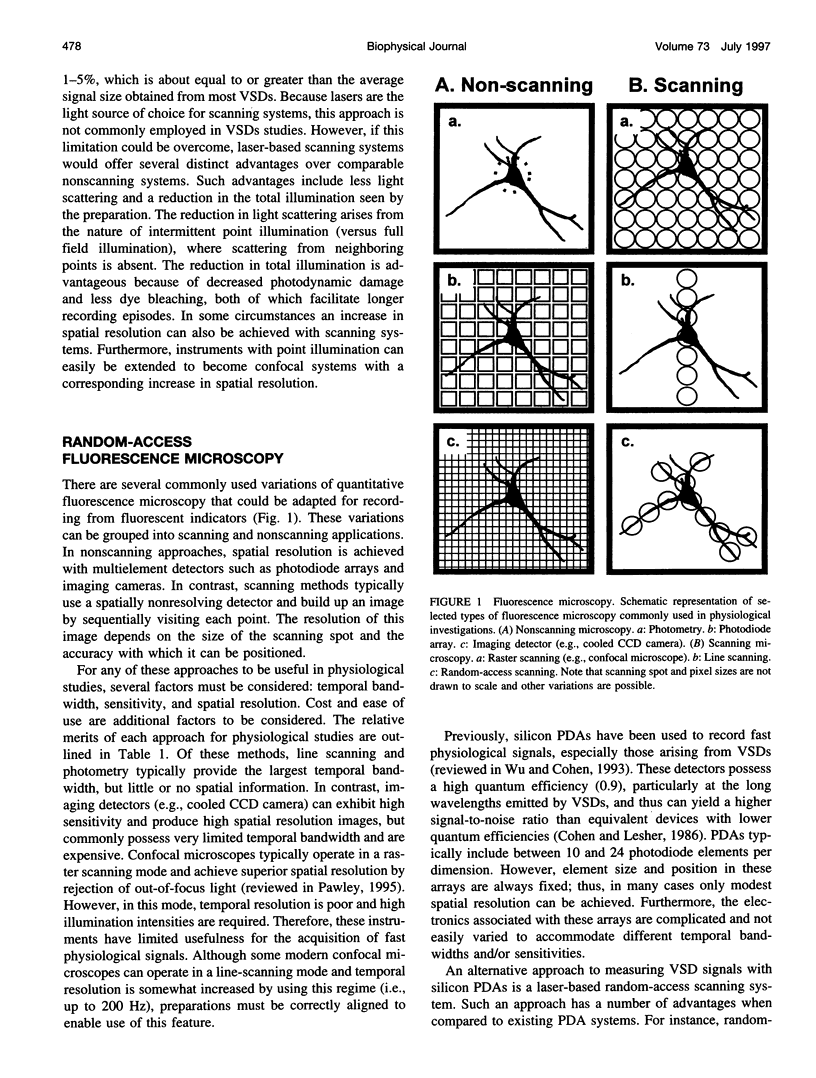
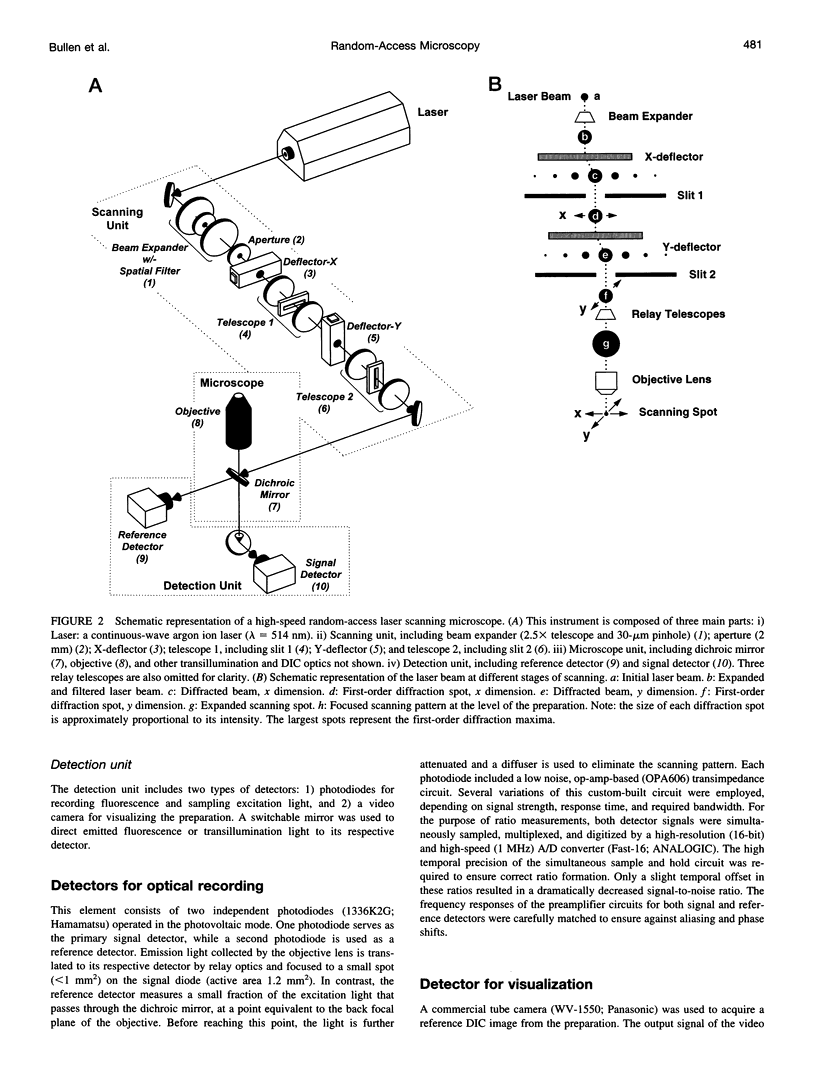
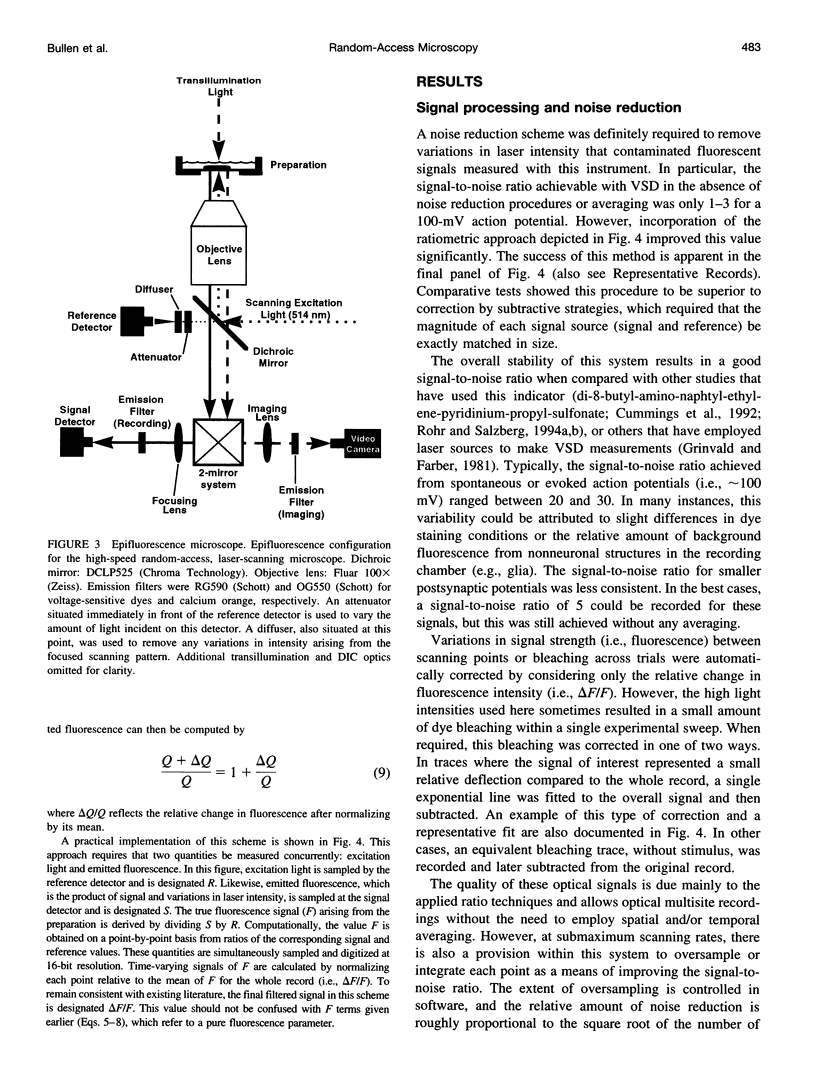
Abstract
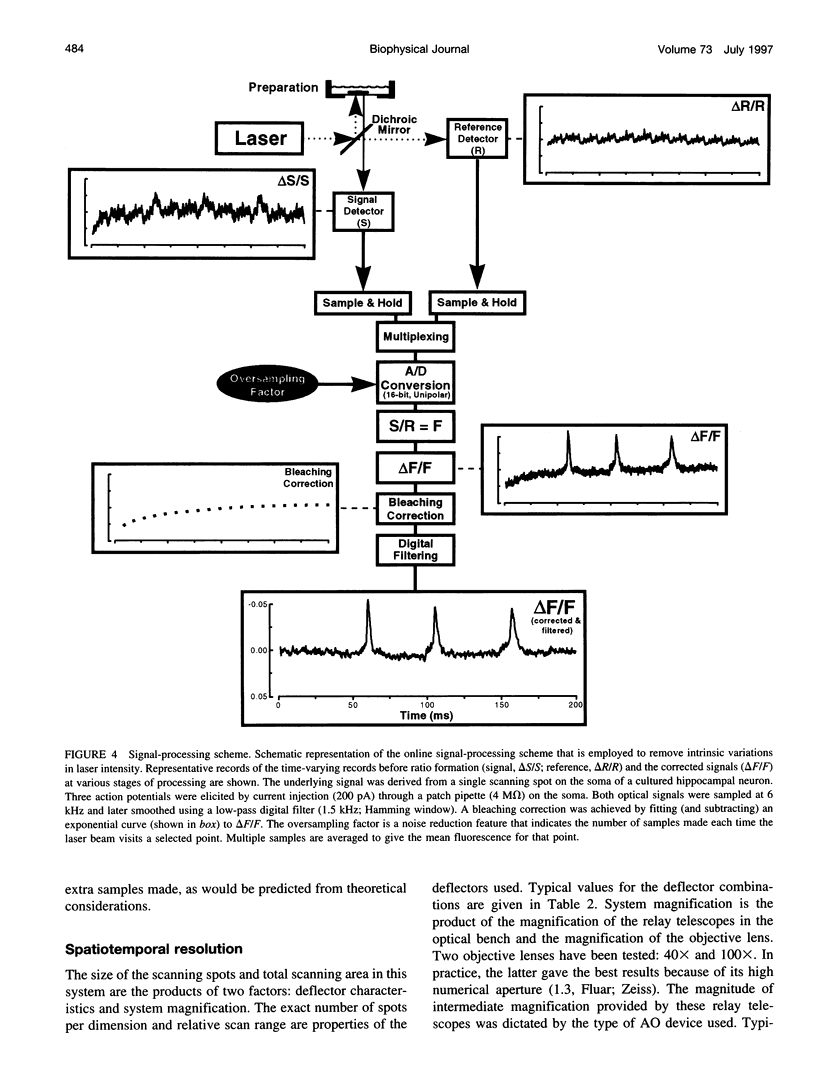
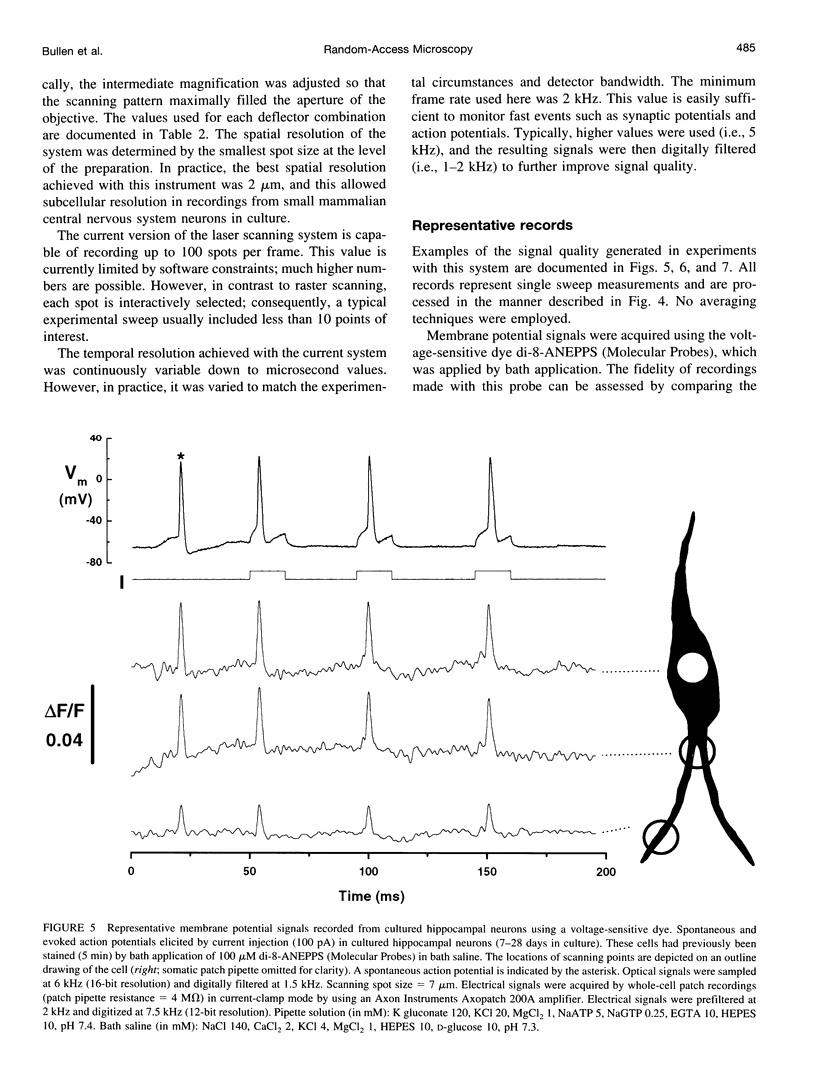
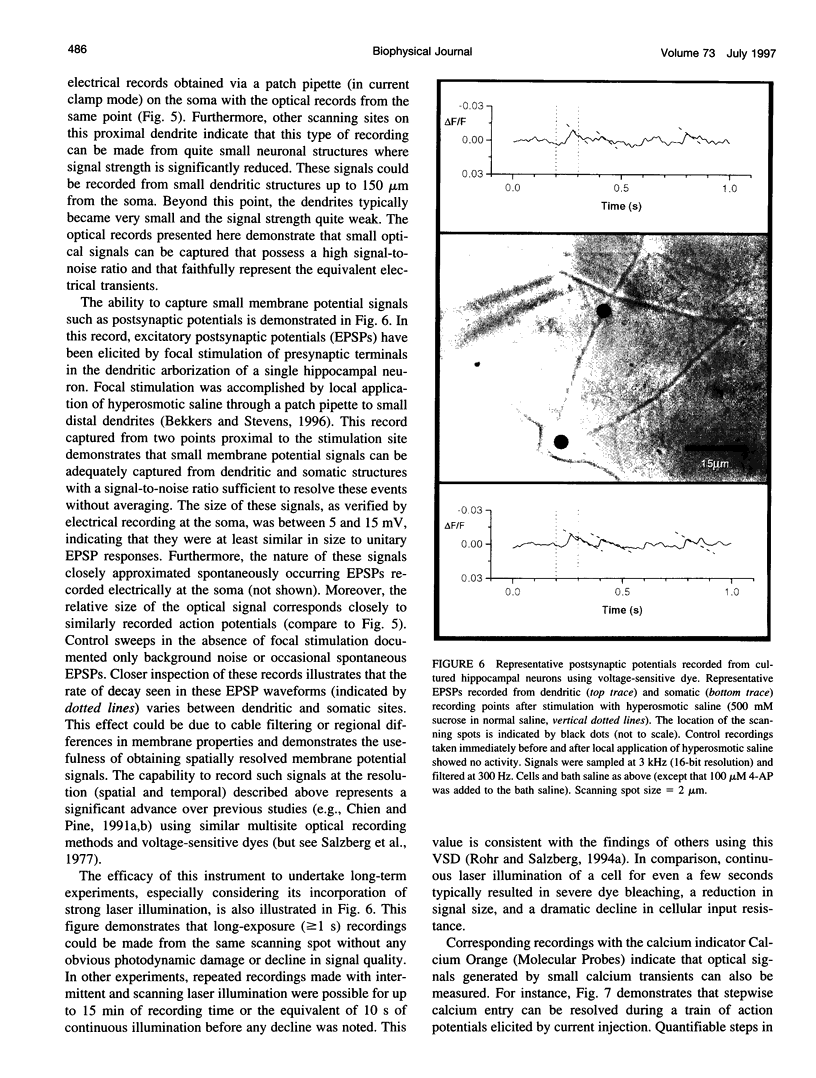
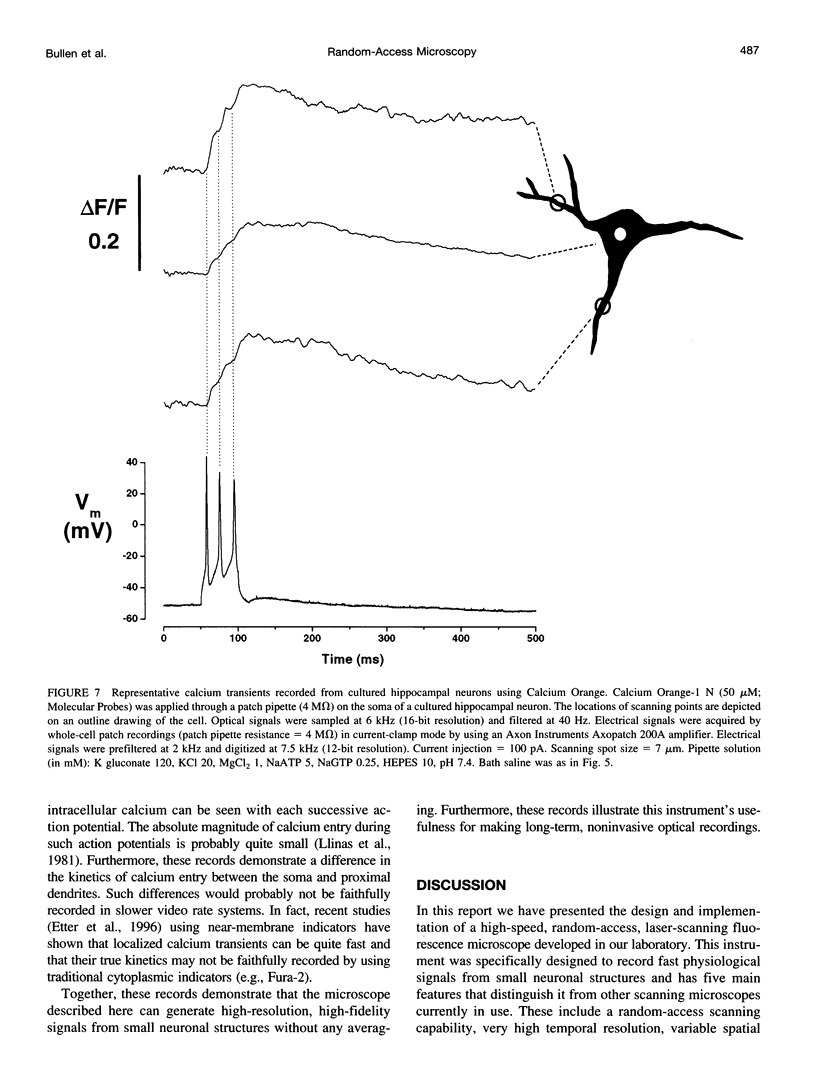
The design and implementation of a high-speed, random-access, laser-scanning fluorescence microscope configured to record fast physiological signals from small neuronal structures with high spatiotemporal resolution is presented. The laser-scanning capability of this nonimaging microscope is provided by two orthogonal acousto-optic deflectors under computer control. Each scanning point can be randomly accessed and has a positioning time of 3-5 microseconds. Sampling time is also computer-controlled and can be varied to maximize the signal-to-noise ratio. Acquisition rates up to 200k samples/s at 16-bit digitizing resolution are possible. The spatial resolution of this instrument is determined by the minimal spot size at the level of the preparation (i.e., 2-7 microns). Scanning points are selected interactively from a reference image collected with differential interference contrast optics and a video camera. Frame rates up to 5 kHz are easily attainable. Intrinsic variations in laser light intensity and scanning spot brightness are overcome by an on-line signal-processing scheme. Representative records obtained with this instrument by using voltage-sensitive dyes and calcium indicators demonstrate the ability to make fast, high-fidelity measurements of membrane potential and intracellular calcium at high spatial resolution (2 microns) without any temporal averaging.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antić S., Zecević D. Optical signals from neurons with internally applied voltage-sensitive dyes. J Neurosci. 1995 Feb;15(2):1392–1405. doi: 10.1523/JNEUROSCI.15-02-01392.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arseni C., Chimion D., Cunescu V., Pietraru N., Salzberg S. Aspecte clinice şi de laborator în boala Kahler. Consideraţii pe marginea a 19 cazuri. Med Interna (Bucur) 1966 Sep;18(9):1049–1057. [PubMed] [Google Scholar]
- Bekkers J. M., Stevens C. F. Cable properties of cultured hippocampal neurons determined from sucrose-evoked miniature EPSCs. J Neurophysiol. 1996 Mar;75(3):1250–1255. doi: 10.1152/jn.1996.75.3.1250. [DOI] [PubMed] [Google Scholar]
- Chien C. B., Pine J. An apparatus for recording synaptic potentials from neuronal cultures using voltage-sensitive fluorescent dyes. J Neurosci Methods. 1991 Jul;38(2-3):93–105. doi: 10.1016/0165-0270(91)90159-w. [DOI] [PubMed] [Google Scholar]
- Chien C. B., Pine J. Voltage-sensitive dye recording of action potentials and synaptic potentials from sympathetic microcultures. Biophys J. 1991 Sep;60(3):697–711. doi: 10.1016/S0006-3495(91)82099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Keynes R. D., Landowne D. Changes in axon light scattering that accompany the action potential: current-dependent components. J Physiol. 1972 Aug;224(3):727–752. doi: 10.1113/jphysiol.1972.sp009920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Lesher S. Optical monitoring of membrane potential: methods of multisite optical measurement. Soc Gen Physiol Ser. 1986;40:71–99. [PubMed] [Google Scholar]
- Cohen L. B., Salzberg B. M., Grinvald A. Optical methods for monitoring neuron activity. Annu Rev Neurosci. 1978;1:171–182. doi: 10.1146/annurev.ne.01.030178.001131. [DOI] [PubMed] [Google Scholar]
- Cohen L., Höpp H. P., Wu J. Y., Xiao C., London J. Optical measurement of action potential activity in invertebrate ganglia. Annu Rev Physiol. 1989;51:527–541. doi: 10.1146/annurev.ph.51.030189.002523. [DOI] [PubMed] [Google Scholar]
- Dillon S., Morad M. A new laser scanning system for measuring action potential propagation in the heart. Science. 1981 Oct 23;214(4519):453–456. doi: 10.1126/science.6974891. [DOI] [PubMed] [Google Scholar]
- Ebner T. J., Chen G. Use of voltage-sensitive dyes and optical recordings in the central nervous system. Prog Neurobiol. 1995 Aug;46(5):463–506. doi: 10.1016/0301-0082(95)00010-s. [DOI] [PubMed] [Google Scholar]
- Etter E. F., Minta A., Poenie M., Fay F. S. Near-membrane [Ca2+] transients resolved using the Ca2+ indicator FFP18. Proc Natl Acad Sci U S A. 1996 May 28;93(11):5368–5373. doi: 10.1073/pnas.93.11.5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S. R., Hubin T., Rosenthal S., Washburn C. A confocal video-rate laser-beam scanning reflected-light microscope with no moving parts. J Microsc. 1990 Jan;157(Pt 1):29–38. doi: 10.1111/j.1365-2818.1990.tb02944.x. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Farber I. C. Optical recording of calcium action potentials from growth cones of cultured neurons with a laser microbeam. Science. 1981 Jun 5;212(4499):1164–1167. doi: 10.1126/science.7233210. [DOI] [PubMed] [Google Scholar]
- Grinvald A. Real-time optical mapping of neuronal activity: from single growth cones to the intact mammalian brain. Annu Rev Neurosci. 1985;8:263–305. doi: 10.1146/annurev.ne.08.030185.001403. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Salzberg B. M., Lev-Ram V., Hildesheim R. Optical recording of synaptic potentials from processes of single neurons using intracellular potentiometric dyes. Biophys J. 1987 Apr;51(4):643–651. doi: 10.1016/S0006-3495(87)83389-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieke E. E., Frostig R. D., Arieli A., Ts'o D. Y., Hildesheim R., Grinvald A. Optical imaging of cortical activity: real-time imaging using extrinsic dye-signals and high resolution imaging based on slow intrinsic-signals. Annu Rev Physiol. 1989;51:543–559. doi: 10.1146/annurev.ph.51.030189.002551. [DOI] [PubMed] [Google Scholar]
- Lieke E. E., Frostig R. D., Arieli A., Ts'o D. Y., Hildesheim R., Grinvald A. Optical imaging of cortical activity: real-time imaging using extrinsic dye-signals and high resolution imaging based on slow intrinsic-signals. Annu Rev Physiol. 1989;51:543–559. doi: 10.1146/annurev.ph.51.030189.002551. [DOI] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Presynaptic calcium currents in squid giant synapse. Biophys J. 1981 Mar;33(3):289–321. doi: 10.1016/S0006-3495(81)84898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loew L. M., Cohen L. B., Dix J., Fluhler E. N., Montana V., Salama G., Wu J. Y. A naphthyl analog of the aminostyryl pyridinium class of potentiometric membrane dyes shows consistent sensitivity in a variety of tissue, cell, and model membrane preparations. J Membr Biol. 1992 Oct;130(1):1–10. doi: 10.1007/BF00233734. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y., Ishiguro Y., Sato T. Optical probing of active membrane potential using lasers and microoptics. Jpn J Physiol. 1993;43 (Suppl 1):S53–S56. [PubMed] [Google Scholar]
- Morad M., Dillon S., Weiss J. An acousto-optically steered laser scanning system for measurement of action potential spread in intact heart. Soc Gen Physiol Ser. 1986;40:211–226. [PubMed] [Google Scholar]
- Rohr S., Salzberg B. M. Characterization of impulse propagation at the microscopic level across geometrically defined expansions of excitable tissue: multiple site optical recording of transmembrane voltage (MSORTV) in patterned growth heart cell cultures. J Gen Physiol. 1994 Aug;104(2):287–309. doi: 10.1085/jgp.104.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr S., Salzberg B. M. Multiple site optical recording of transmembrane voltage (MSORTV) in patterned growth heart cell cultures: assessing electrical behavior, with microsecond resolution, on a cellular and subcellular scale. Biophys J. 1994 Sep;67(3):1301–1315. doi: 10.1016/S0006-3495(94)80602-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W. N., Reichardt L. F. Species-specific effects on the optical signals of voltage-sensitive dyes. J Membr Biol. 1979 Aug;48(4):343–356. doi: 10.1007/BF01869445. [DOI] [PubMed] [Google Scholar]
- Salzberg B. M., Grinvald A., Cohen L. B., Davila H. V., Ross W. N. Optical recording of neuronal activity in an invertebrate central nervous system: simultaneous monitoring of several neurons. J Neurophysiol. 1977 Nov;40(6):1281–1291. doi: 10.1152/jn.1977.40.6.1281. [DOI] [PubMed] [Google Scholar]
- Sinha S. R., Patel S. S., Saggau P. Simultaneous optical recording of evoked and spontaneous transients of membrane potential and intracellular calcium concentration with high spatio-temporal resolution. J Neurosci Methods. 1995 Aug;60(1-2):49–60. doi: 10.1016/0165-0270(94)00219-7. [DOI] [PubMed] [Google Scholar]
- Sutor B., Hablitz J. J., Rucker F., ten Bruggencate G. Spread of epileptiform activity in the immature rat neocortex studied with voltage-sensitive dyes and laser scanning microscopy. J Neurophysiol. 1994 Oct;72(4):1756–1768. doi: 10.1152/jn.1994.72.4.1756. [DOI] [PubMed] [Google Scholar]
- Zecević D. Multiple spike-initiation zones in single neurons revealed by voltage-sensitive dyes. Nature. 1996 May 23;381(6580):322–325. doi: 10.1038/381322a0. [DOI] [PubMed] [Google Scholar]



