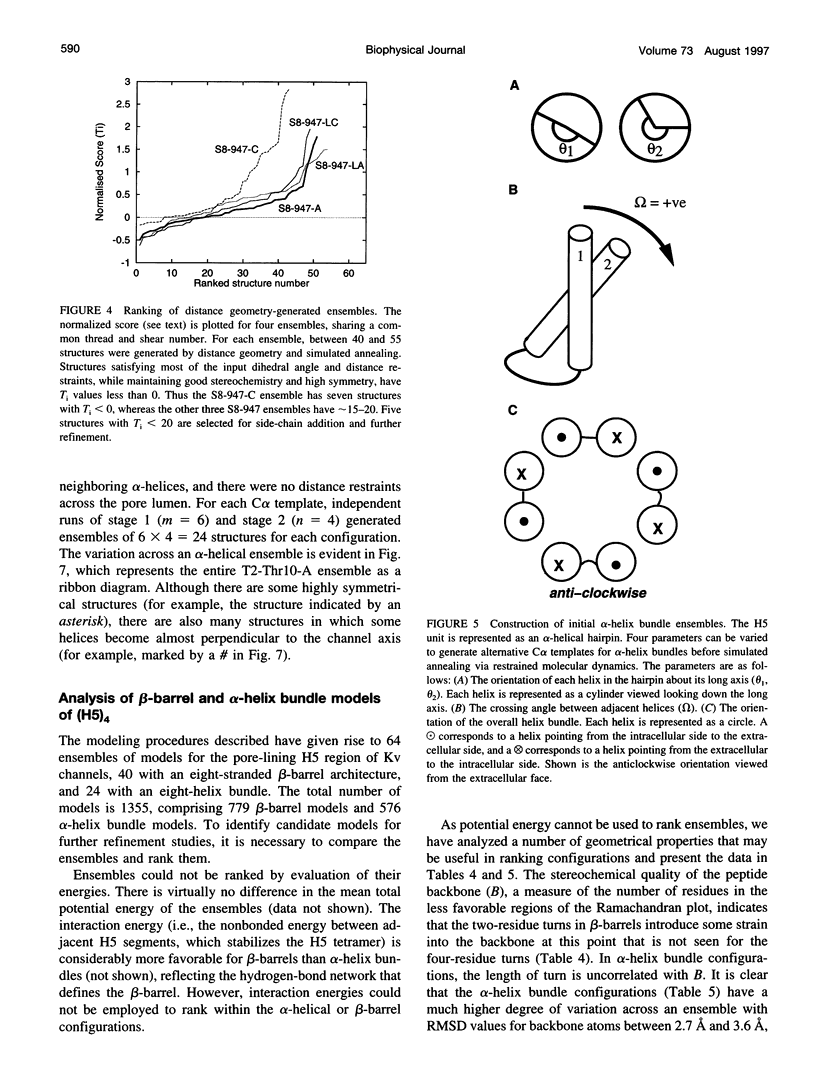
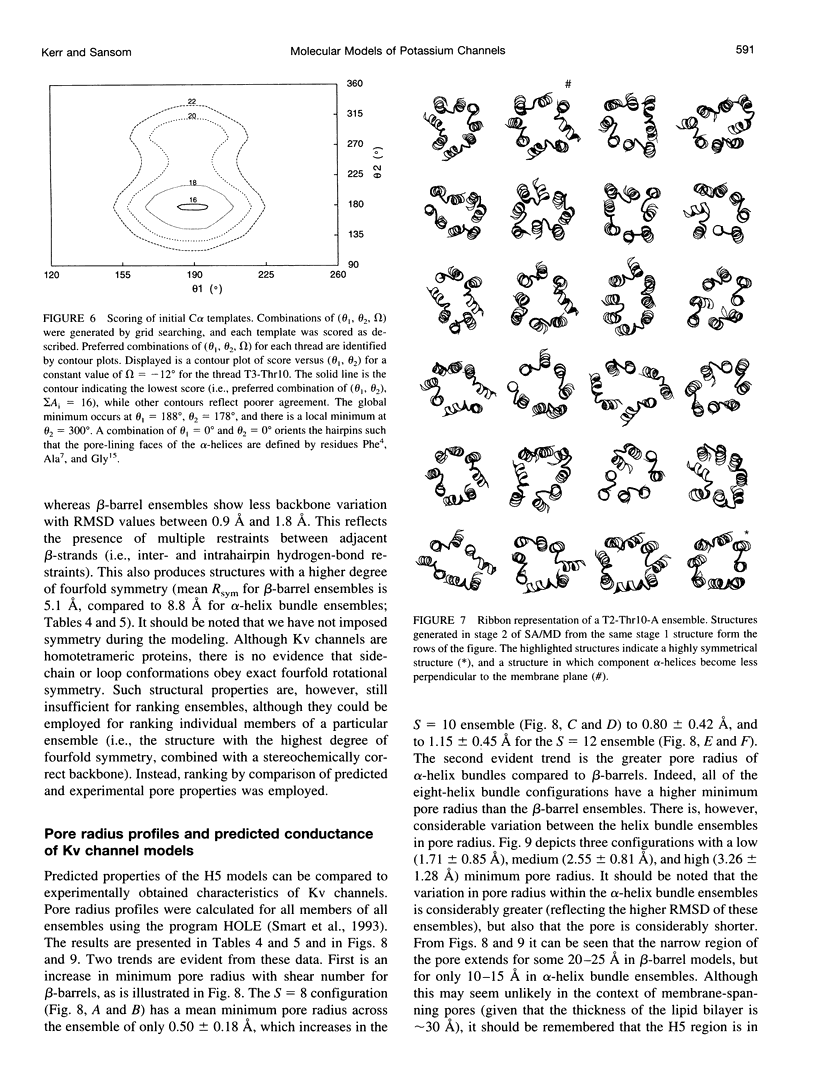
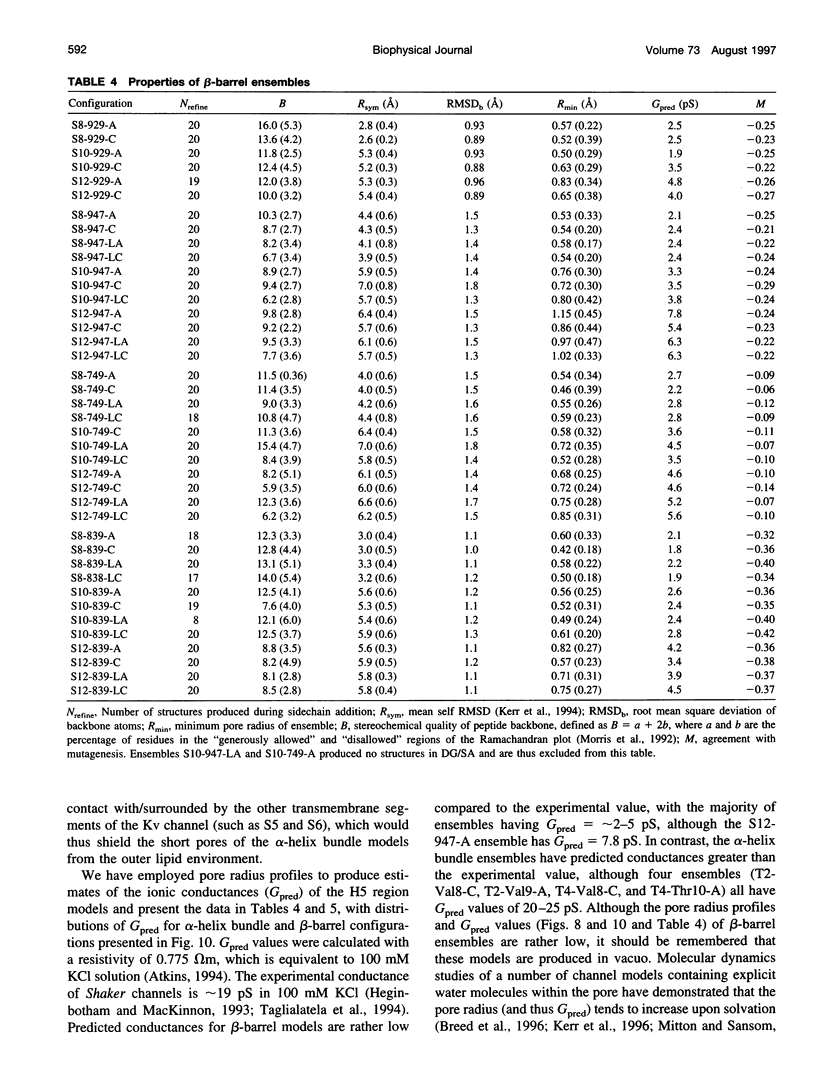
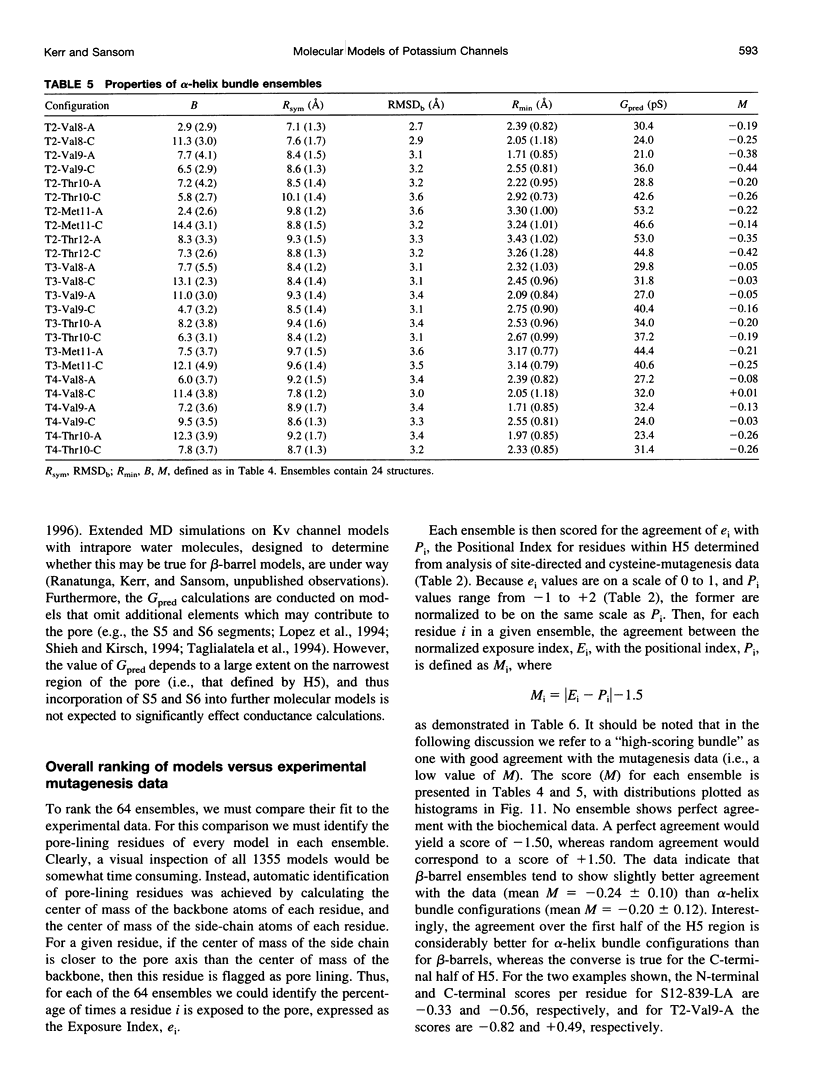
Abstract
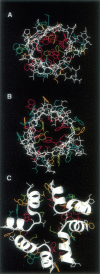
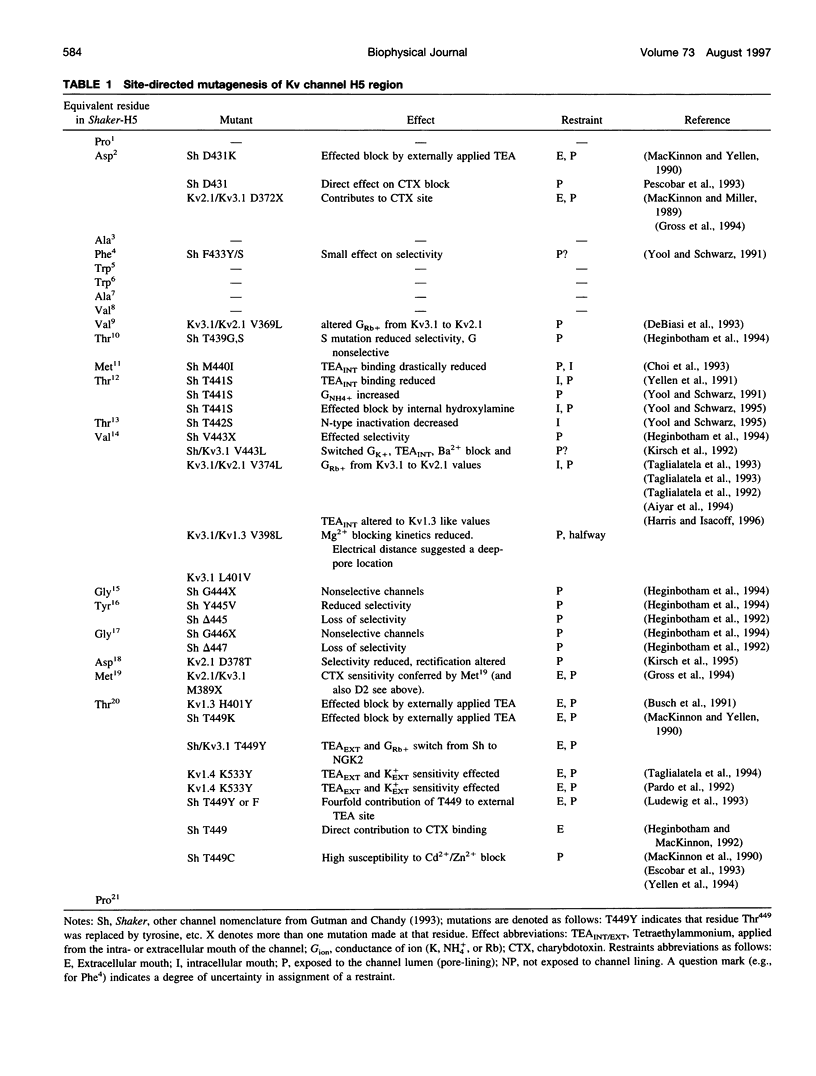
Although there is a large body of site-directed mutagenesis data that identify the pore-lining sequence of the voltage-gated potassium channel, the structure of this region remains unknown. We have interpreted the available biochemical data as a set of topological and orientational restraints and employed these restraints to produce molecular models of the potassium channel pore region, H5. The H5 sequence has been modeled either as a tetramer of membrane-spanning beta-hairpins, thus producing an eight-stranded beta-barrel, or as a tetramer of incompletely membrane-spanning alpha-helical hairpins, thus producing an eight-staved alpha-helix bundle. In total, restraints-directed modeling has produced 40 different configurations of the beta-barrel model, each configuration comprising an ensemble of 20 structures, and 24 different configurations of the alpha-helix bundle model, each comprising an ensemble of 24 structures. Thus, over 1300 model structures for H5 have been generated. Configurations have been ranked on the basis of their predicted pore properties and on the extent of their agreement with the biochemical data. This ranking is employed to identify particular configurations of H5 that may be explored further as models of the pore-lining region of the voltage-gated potassium channel pore.
Full text
PDF


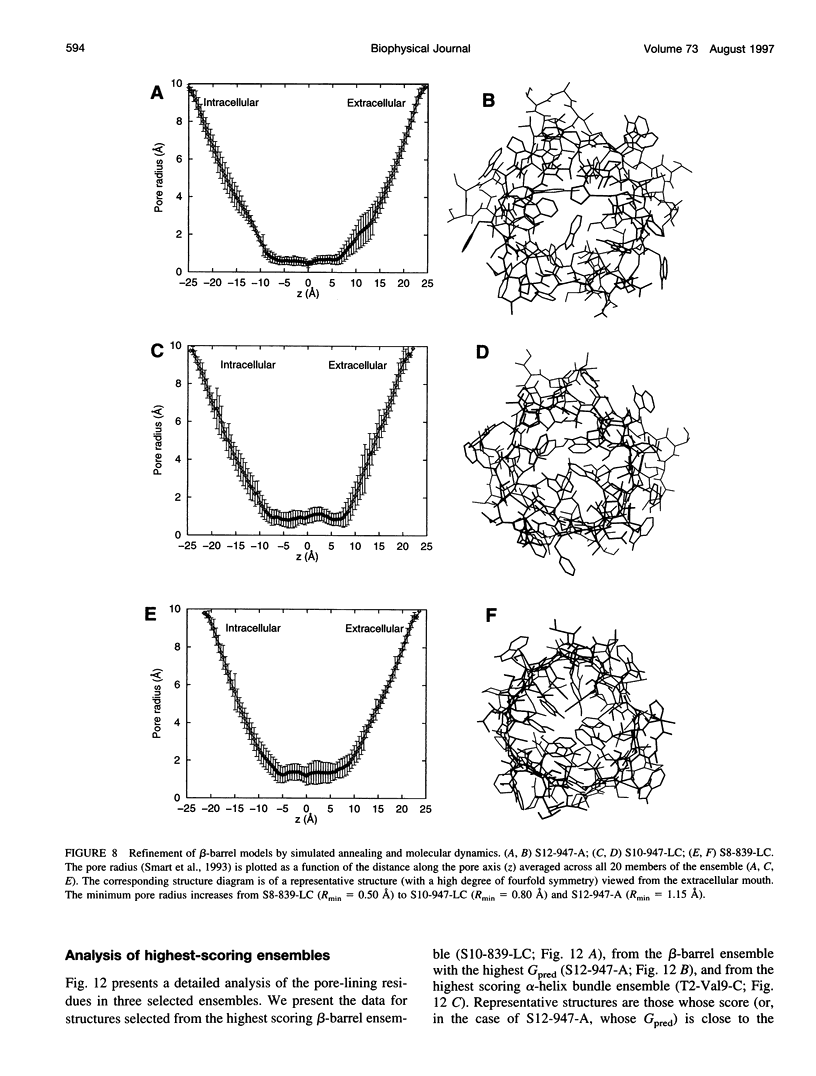
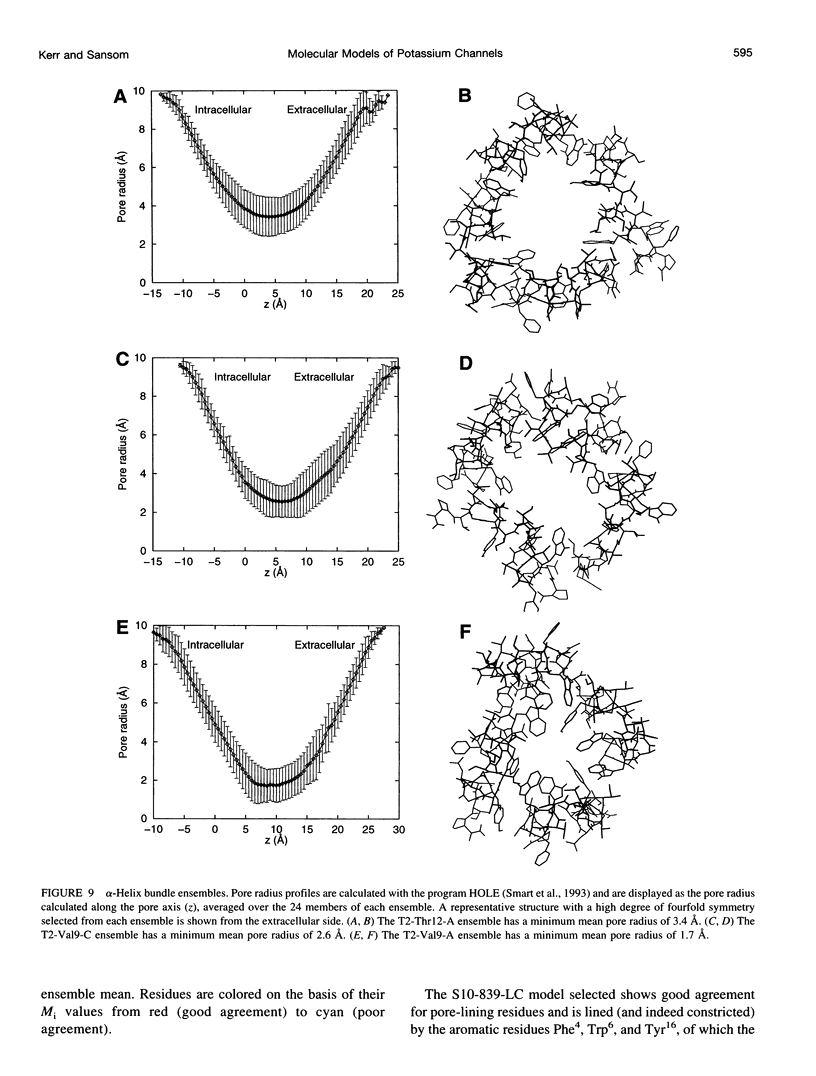
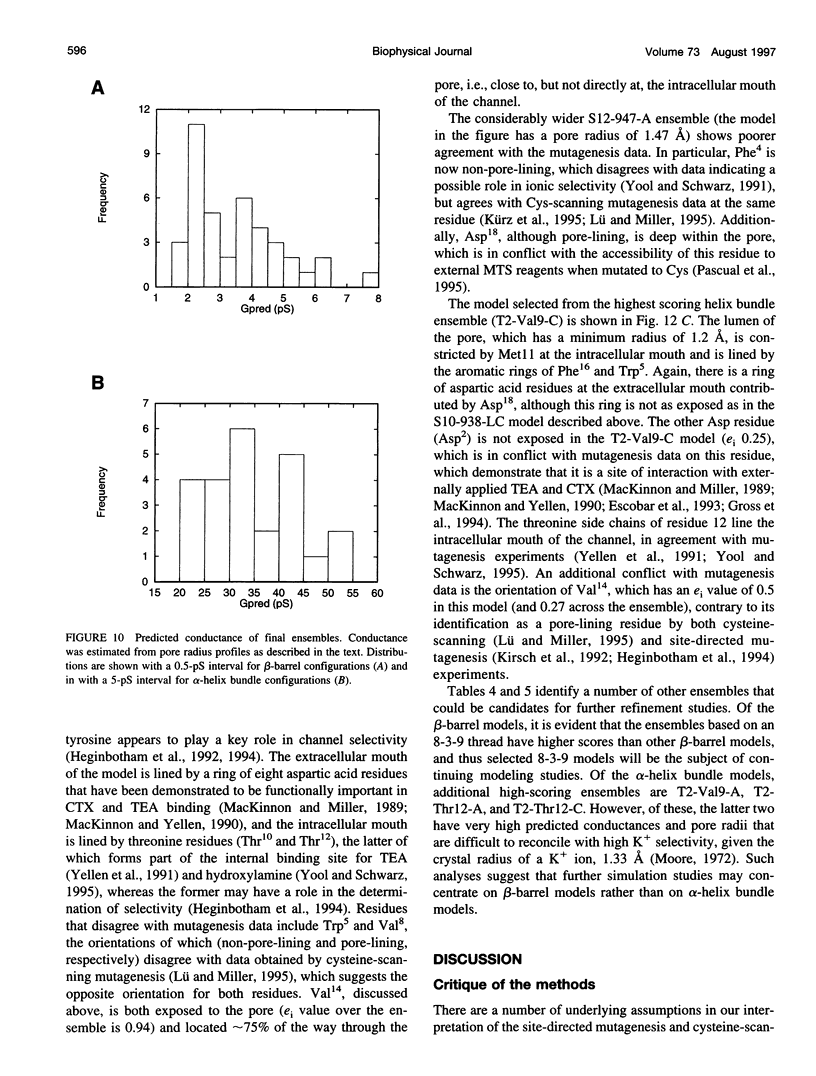
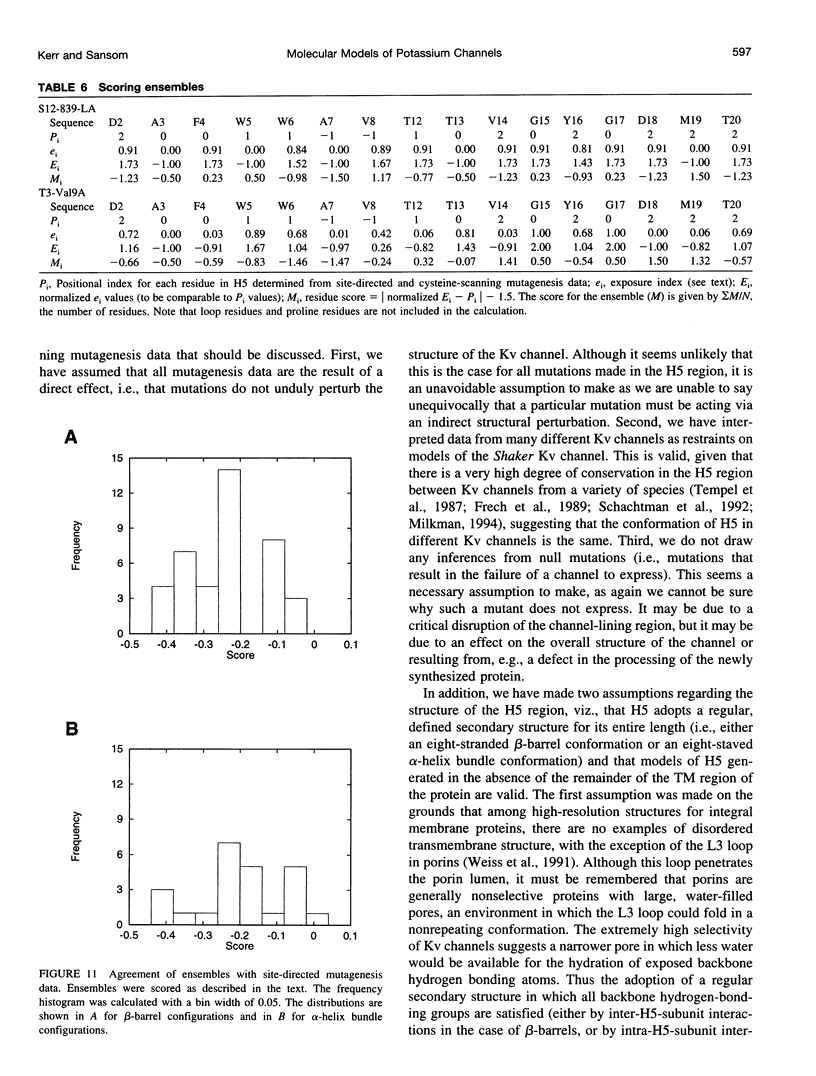




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiyar J., Nguyen A. N., Chandy K. G., Grissmer S. The P-region and S6 of Kv3.1 contribute to the formation of the ion conduction pathway. Biophys J. 1994 Dec;67(6):2261–2264. doi: 10.1016/S0006-3495(94)80710-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich R. Potassium channels. Advent of a new family. Nature. 1993 Mar 11;362(6416):107–108. doi: 10.1038/362107a0. [DOI] [PubMed] [Google Scholar]
- Antz C., Geyer M., Fakler B., Schott M. K., Guy H. R., Frank R., Ruppersberg J. P., Kalbitzer H. R. NMR structure of inactivation gates from mammalian voltage-dependent potassium channels. Nature. 1997 Jan 16;385(6613):272–275. doi: 10.1038/385272a0. [DOI] [PubMed] [Google Scholar]
- Baker E. N., Hubbard R. E. Hydrogen bonding in globular proteins. Prog Biophys Mol Biol. 1984;44(2):97–179. doi: 10.1016/0079-6107(84)90007-5. [DOI] [PubMed] [Google Scholar]
- Bogusz S., Boxer A., Busath D. D. An SS1-SS2 beta-barrel structure for the voltage-activated potassium channel. Protein Eng. 1992 Jun;5(4):285–293. doi: 10.1093/protein/5.4.285. [DOI] [PubMed] [Google Scholar]
- Bradley J. C., Richards W. G. Potassium channels: a computer prediction of structure and selectivity. Protein Eng. 1994 Jul;7(7):859–862. doi: 10.1093/protein/7.7.859. [DOI] [PubMed] [Google Scholar]
- Breed J., Biggin P. C., Kerr I. D., Smart O. S., Sansom M. S. Alamethicin channels - modelling via restrained molecular dynamics simulations. Biochim Biophys Acta. 1997 Apr 26;1325(2):235–249. doi: 10.1016/s0005-2736(96)00262-3. [DOI] [PubMed] [Google Scholar]
- Breed J., Kerr I. D., Sankararamakrishnan R., Sansom M. S. Packing interactions of Aib-containing helices: molecular modeling of parallel dimers of simple hydrophobic helices and of alamethicin. Biopolymers. 1995 Jun;35(6):639–655. doi: 10.1002/bip.360350610. [DOI] [PubMed] [Google Scholar]
- Breed J., Sankararamakrishnan R., Kerr I. D., Sansom M. S. Molecular dynamics simulations of water within models of ion channels. Biophys J. 1996 Apr;70(4):1643–1661. doi: 10.1016/S0006-3495(96)79727-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M. Functional bases for interpreting amino acid sequences of voltage-dependent K+ channels. Annu Rev Biophys Biomol Struct. 1993;22:173–198. doi: 10.1146/annurev.bb.22.060193.001133. [DOI] [PubMed] [Google Scholar]
- Busch A. E., Hurst R. S., North R. A., Adelman J. P., Kavanaugh M. P. Current inactivation involves a histidine residue in the pore of the rat lymphocyte potassium channel RGK5. Biochem Biophys Res Commun. 1991 Sep 30;179(3):1384–1390. doi: 10.1016/0006-291x(91)91726-s. [DOI] [PubMed] [Google Scholar]
- Choi K. L., Mossman C., Aubé J., Yellen G. The internal quaternary ammonium receptor site of Shaker potassium channels. Neuron. 1993 Mar;10(3):533–541. doi: 10.1016/0896-6273(93)90340-w. [DOI] [PubMed] [Google Scholar]
- Cosette P., Brachais L., Bernardi E., Duclohier H. Investigating synthetic P-regions from voltage-gated sodium channel at the conformational and functional levels. Eur Biophys J. 1997;25(4):275–284. doi: 10.1007/s002490050039. [DOI] [PubMed] [Google Scholar]
- Cowan S. W., Schirmer T., Rummel G., Steiert M., Ghosh R., Pauptit R. A., Jansonius J. N., Rosenbusch J. P. Crystal structures explain functional properties of two E. coli porins. Nature. 1992 Aug 27;358(6389):727–733. doi: 10.1038/358727a0. [DOI] [PubMed] [Google Scholar]
- De Biasi M., Hartmann H. A., Drewe J. A., Taglialatela M., Brown A. M., Kirsch G. E. Inactivation determined by a single site in K+ pores. Pflugers Arch. 1993 Jan;422(4):354–363. doi: 10.1007/BF00374291. [DOI] [PubMed] [Google Scholar]
- Donnelly D., Overington J. P., Ruffle S. V., Nugent J. H., Blundell T. L. Modeling alpha-helical transmembrane domains: the calculation and use of substitution tables for lipid-facing residues. Protein Sci. 1993 Jan;2(1):55–70. doi: 10.1002/pro.5560020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupnik C. A., Davidson N., Lester H. A. The inward rectifier potassium channel family. Curr Opin Neurobiol. 1995 Jun;5(3):268–277. doi: 10.1016/0959-4388(95)80038-7. [DOI] [PubMed] [Google Scholar]
- Durell S. R., Guy H. R. Atomic scale structure and functional models of voltage-gated potassium channels. Biophys J. 1992 Apr;62(1):238–250. doi: 10.1016/S0006-3495(92)81809-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durell S. R., Guy H. R. Structural model of the outer vestibule and selectivity filter of the Shaker voltage-gated K+ channel. Neuropharmacology. 1996;35(7):761–773. doi: 10.1016/0028-3908(96)00097-4. [DOI] [PubMed] [Google Scholar]
- Escobar L., Root M. J., MacKinnon R. Influence of protein surface charge on the bimolecular kinetics of a potassium channel peptide inhibitor. Biochemistry. 1993 Jul 13;32(27):6982–6987. doi: 10.1021/bi00078a024. [DOI] [PubMed] [Google Scholar]
- Frech G. C., VanDongen A. M., Schuster G., Brown A. M., Joho R. H. A novel potassium channel with delayed rectifier properties isolated from rat brain by expression cloning. Nature. 1989 Aug 24;340(6235):642–645. doi: 10.1038/340642a0. [DOI] [PubMed] [Google Scholar]
- Gross A., Abramson T., MacKinnon R. Transfer of the scorpion toxin receptor to an insensitive potassium channel. Neuron. 1994 Oct;13(4):961–966. doi: 10.1016/0896-6273(94)90261-5. [DOI] [PubMed] [Google Scholar]
- Haris P. I., Ramesh B., Sansom M. S., Kerr I. D., Srai K. S., Chapman D. Studies of the pore-forming domain of a voltage-gated potassium channel protein. Protein Eng. 1994 Feb;7(2):255–262. doi: 10.1093/protein/7.2.255. [DOI] [PubMed] [Google Scholar]
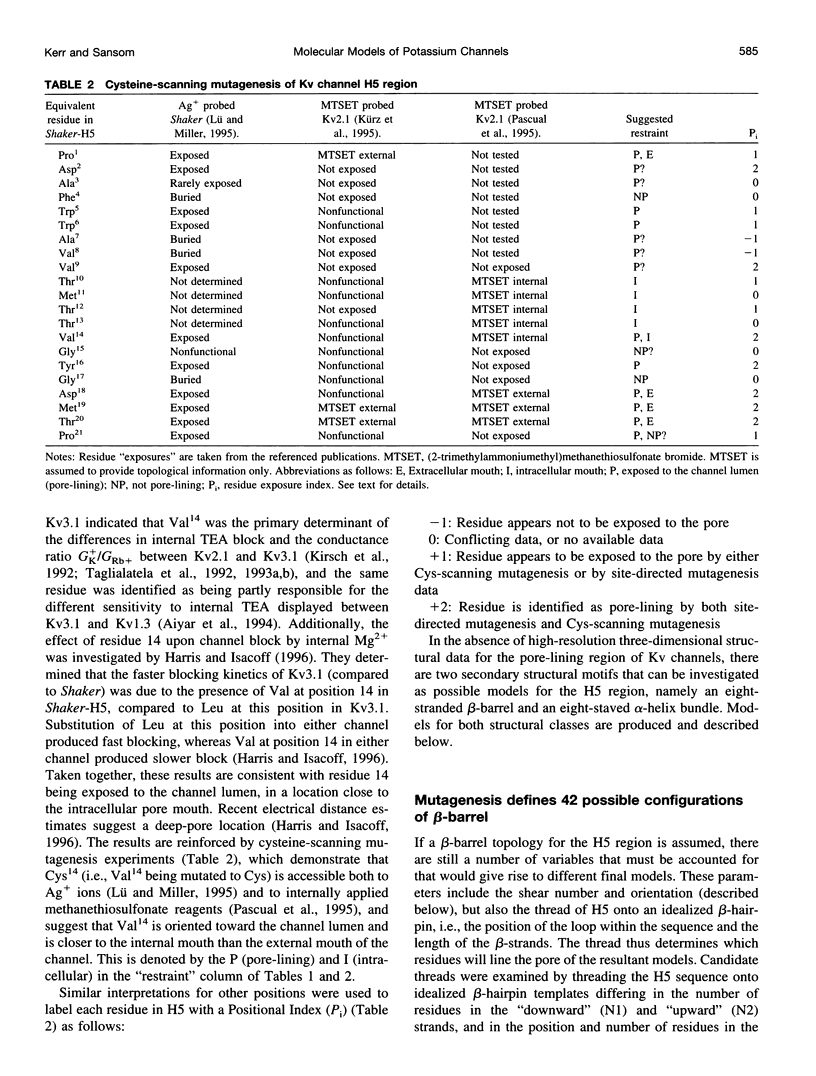
- Harris R. E., Isacoff E. Y. Hydrophobic mutations alter the movement of Mg2+ in the pore of voltage-gated potassium channels. Biophys J. 1996 Jul;71(1):209–219. doi: 10.1016/S0006-3495(96)79217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann H. A., Kirsch G. E., Drewe J. A., Taglialatela M., Joho R. H., Brown A. M. Exchange of conduction pathways between two related K+ channels. Science. 1991 Feb 22;251(4996):942–944. doi: 10.1126/science.2000495. [DOI] [PubMed] [Google Scholar]
- Heginbotham L., Abramson T., MacKinnon R. A functional connection between the pores of distantly related ion channels as revealed by mutant K+ channels. Science. 1992 Nov 13;258(5085):1152–1155. doi: 10.1126/science.1279807. [DOI] [PubMed] [Google Scholar]
- Heginbotham L., Lu Z., Abramson T., MacKinnon R. Mutations in the K+ channel signature sequence. Biophys J. 1994 Apr;66(4):1061–1067. doi: 10.1016/S0006-3495(94)80887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heginbotham L., MacKinnon R. Conduction properties of the cloned Shaker K+ channel. Biophys J. 1993 Nov;65(5):2089–2096. doi: 10.1016/S0006-3495(93)81244-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heginbotham L., MacKinnon R. The aromatic binding site for tetraethylammonium ion on potassium channels. Neuron. 1992 Mar;8(3):483–491. doi: 10.1016/0896-6273(92)90276-j. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Herzyk P., Hubbard R. E. Automated method for modeling seven-helix transmembrane receptors from experimental data. Biophys J. 1995 Dec;69(6):2419–2442. doi: 10.1016/S0006-3495(95)80112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho K., Nichols C. G., Lederer W. J., Lytton J., Vassilev P. M., Kanazirska M. V., Hebert S. C. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature. 1993 Mar 4;362(6415):31–38. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- Hucho F., Tsetlin V. I., Machold J. The emerging three-dimensional structure of a receptor. The nicotinic acetylcholine receptor. Eur J Biochem. 1996 Aug 1;239(3):539–557. doi: 10.1111/j.1432-1033.1996.0539u.x. [DOI] [PubMed] [Google Scholar]
- Iwata S., Ostermeier C., Ludwig B., Michel H. Structure at 2.8 A resolution of cytochrome c oxidase from Paracoccus denitrificans. Nature. 1995 Aug 24;376(6542):660–669. doi: 10.1038/376660a0. [DOI] [PubMed] [Google Scholar]
- Jin A. Y., Weaver D. F. A computational model of the HBK2 potassium channel ion pore. Biochem Biophys Res Commun. 1993 Aug 16;194(3):1117–1123. doi: 10.1006/bbrc.1993.1937. [DOI] [PubMed] [Google Scholar]
- Jin L., Cohen F. E., Wells J. A. Structure from function: screening structural models with functional data. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):113–117. doi: 10.1073/pnas.91.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. D., Doak D. G., Sankararamakrishnan R., Breed J., Sansom M. S. Molecular modelling of Staphylococcal delta-toxin ion channels by restrained molecular dynamics. Protein Eng. 1996 Feb;9(2):161–171. doi: 10.1093/protein/9.2.161. [DOI] [PubMed] [Google Scholar]
- Kerr I. D., Sankararamakrishnan R., Smart O. S., Sansom M. S. Parallel helix bundles and ion channels: molecular modeling via simulated annealing and restrained molecular dynamics. Biophys J. 1994 Oct;67(4):1501–1515. doi: 10.1016/S0006-3495(94)80624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketchum K. A., Joiner W. J., Sellers A. J., Kaczmarek L. K., Goldstein S. A. A new family of outwardly rectifying potassium channel proteins with two pore domains in tandem. Nature. 1995 Aug 24;376(6542):690–695. doi: 10.1038/376690a0. [DOI] [PubMed] [Google Scholar]
- Kirsch G. E., Drewe J. A., Taglialatela M., Joho R. H., DeBiasi M., Hartmann H. A., Brown A. M. A single nonpolar residue in the deep pore of related K+ channels acts as a K+:Rb+ conductance switch. Biophys J. 1992 Apr;62(1):136–144. doi: 10.1016/S0006-3495(92)81800-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch G. E., Pascual J. M., Shieh C. C. Functional role of a conserved aspartate in the external mouth of voltage-gated potassium channels. Biophys J. 1995 May;68(5):1804–1813. doi: 10.1016/S0006-3495(95)80357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo Y., Baldwin T. J., Jan Y. N., Jan L. Y. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature. 1993 Mar 11;362(6416):127–133. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- Kuntz I. D., Thomason J. F., Oshiro C. M. Distance geometry. Methods Enzymol. 1989;177:159–204. doi: 10.1016/0076-6879(89)77011-7. [DOI] [PubMed] [Google Scholar]
- Kürz L. L., Zühlke R. D., Zhang H. J., Joho R. H. Side-chain accessibilities in the pore of a K+ channel probed by sulfhydryl-specific reagents after cysteine-scanning mutagenesis. Biophys J. 1995 Mar;68(3):900–905. doi: 10.1016/S0006-3495(95)80266-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage F., Guillemare E., Fink M., Duprat F., Lazdunski M., Romey G., Barhanin J. TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO J. 1996 Mar 1;15(5):1004–1011. [PMC free article] [PubMed] [Google Scholar]
- Li M., Unwin N., Stauffer K. A., Jan Y. N., Jan L. Y. Images of purified Shaker potassium channels. Curr Biol. 1994 Feb 1;4(2):110–115. doi: 10.1016/s0960-9822(94)00026-6. [DOI] [PubMed] [Google Scholar]
- Lipkind G. M., Hanck D. A., Fozzard H. A. A structural motif for the voltage-gated potassium channel pore. Proc Natl Acad Sci U S A. 1995 Sep 26;92(20):9215–9219. doi: 10.1073/pnas.92.20.9215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez G. A., Jan Y. N., Jan L. Y. Evidence that the S6 segment of the Shaker voltage-gated K+ channel comprises part of the pore. Nature. 1994 Jan 13;367(6459):179–182. doi: 10.1038/367179a0. [DOI] [PubMed] [Google Scholar]
- Ludewig U., Lorra C., Pongs O., Heinemann S. H. A site accessible to extracellular TEA+ and K+ influences intracellular Mg2+ block of cloned potassium channels. Eur Biophys J. 1993;22(4):237–247. doi: 10.1007/BF00180258. [DOI] [PubMed] [Google Scholar]
- Lund O., Hansen J., Brunak S., Bohr J. Relationship between protein structure and geometrical constraints. Protein Sci. 1996 Nov;5(11):2217–2225. doi: 10.1002/pro.5560051108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü Q., Miller C. Silver as a probe of pore-forming residues in a potassium channel. Science. 1995 Apr 14;268(5208):304–307. doi: 10.1126/science.7716526. [DOI] [PubMed] [Google Scholar]
- MacKinnon R. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature. 1991 Mar 21;350(6315):232–235. doi: 10.1038/350232a0. [DOI] [PubMed] [Google Scholar]
- MacKinnon R., Heginbotham L., Abramson T. Mapping the receptor site for charybdotoxin, a pore-blocking potassium channel inhibitor. Neuron. 1990 Dec;5(6):767–771. doi: 10.1016/0896-6273(90)90335-d. [DOI] [PubMed] [Google Scholar]
- MacKinnon R., Miller C. Mutant potassium channels with altered binding of charybdotoxin, a pore-blocking peptide inhibitor. Science. 1989 Sep 22;245(4924):1382–1385. doi: 10.1126/science.2476850. [DOI] [PubMed] [Google Scholar]
- MacKinnon R., Yellen G. Mutations affecting TEA blockade and ion permeation in voltage-activated K+ channels. Science. 1990 Oct 12;250(4978):276–279. doi: 10.1126/science.2218530. [DOI] [PubMed] [Google Scholar]
- Milkman R. An Escherichia coli homologue of eukaryotic potassium channel proteins. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3510–3514. doi: 10.1073/pnas.91.9.3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A. L., MacArthur M. W., Hutchinson E. G., Thornton J. M. Stereochemical quality of protein structure coordinates. Proteins. 1992 Apr;12(4):345–364. doi: 10.1002/prot.340120407. [DOI] [PubMed] [Google Scholar]
- Murzin A. G., Lesk A. M., Chothia C. Principles determining the structure of beta-sheet barrels in proteins. I. A theoretical analysis. J Mol Biol. 1994 Mar 11;236(5):1369–1381. doi: 10.1016/0022-2836(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Naranjo D., Miller C. A strongly interacting pair of residues on the contact surface of charybdotoxin and a Shaker K+ channel. Neuron. 1996 Jan;16(1):123–130. doi: 10.1016/s0896-6273(00)80029-x. [DOI] [PubMed] [Google Scholar]
- Nilges M., Brünger A. T. Automated modeling of coiled coils: application to the GCN4 dimerization region. Protein Eng. 1991 Aug;4(6):649–659. doi: 10.1093/protein/4.6.649. [DOI] [PubMed] [Google Scholar]
- Pardo L. A., Heinemann S. H., Terlau H., Ludewig U., Lorra C., Pongs O., Stühmer W. Extracellular K+ specifically modulates a rat brain K+ channel. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2466–2470. doi: 10.1073/pnas.89.6.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual J. M., Shieh C. C., Kirsch G. E., Brown A. M. Multiple residues specify external tetraethylammonium blockade in voltage-gated potassium channels. Biophys J. 1995 Aug;69(2):428–434. doi: 10.1016/S0006-3495(95)79915-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peled H., Shai Y. Membrane interaction and self-assembly within phospholipid membranes of synthetic segments corresponding to the H-5 region of the shaker K+ channel. Biochemistry. 1993 Aug 10;32(31):7879–7885. doi: 10.1021/bi00082a007. [DOI] [PubMed] [Google Scholar]
- Pongs O. Molecular biology of voltage-dependent potassium channels. Physiol Rev. 1992 Oct;72(4 Suppl):S69–S88. doi: 10.1152/physrev.1992.72.suppl_4.S69. [DOI] [PubMed] [Google Scholar]
- Pérez-Cornejo P., Begenisich T. The multi-ion nature of the pore in Shaker K+ channels. Biophys J. 1994 Jun;66(6):1929–1938. doi: 10.1016/S0006-3495(94)80986-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux B. Valence selectivity of the gramicidin channel: a molecular dynamics free energy perturbation study. Biophys J. 1996 Dec;71(6):3177–3185. doi: 10.1016/S0006-3495(96)79511-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankararamakrishnan R., Adcock C., Sansom M. S. The pore domain of the nicotinic acetylcholine receptor: molecular modeling, pore dimensions, and electrostatics. Biophys J. 1996 Oct;71(4):1659–1671. doi: 10.1016/S0006-3495(96)79370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom M. S., Kerr I. D. Transbilayer pores formed by beta-barrels: molecular modeling of pore structures and properties. Biophys J. 1995 Oct;69(4):1334–1343. doi: 10.1016/S0006-3495(95)80000-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom M. S., Sankararamakrishnan R., Kerr I. D. Modelling membrane proteins using structural restraints. Nat Struct Biol. 1995 Aug;2(8):624–631. doi: 10.1038/nsb0895-624. [DOI] [PubMed] [Google Scholar]
- Schachtman D. P., Schroeder J. I., Lucas W. J., Anderson J. A., Gaber R. F. Expression of an inward-rectifying potassium channel by the Arabidopsis KAT1 cDNA. Science. 1992 Dec 4;258(5088):1654–1658. doi: 10.1126/science.8966547. [DOI] [PubMed] [Google Scholar]
- Shieh C. C., Kirsch G. E. Mutational analysis of ion conduction and drug binding sites in the inner mouth of voltage-gated K+ channels. Biophys J. 1994 Dec;67(6):2316–2325. doi: 10.1016/S0006-3495(94)80718-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Anzai K., Kirino Y., Lee S., Aoyagi H. Ion channel activity of a synthetic peptide with a primary structure corresponding to the presumed pore-forming region of the voltage dependent potassium channel. Biochem Biophys Res Commun. 1994 Jan 28;198(2):445–450. doi: 10.1006/bbrc.1994.1065. [DOI] [PubMed] [Google Scholar]
- Sibanda B. L., Blundell T. L., Thornton J. M. Conformation of beta-hairpins in protein structures. A systematic classification with applications to modelling by homology, electron density fitting and protein engineering. J Mol Biol. 1989 Apr 20;206(4):759–777. doi: 10.1016/0022-2836(89)90583-4. [DOI] [PubMed] [Google Scholar]
- Singh C., Sankararamakrishnan R., Subramaniam S., Jakobsson E. Solvation, water permeation, and ionic selectivity of a putative model for the pore region of the voltage-gated sodium channel. Biophys J. 1996 Nov;71(5):2276–2288. doi: 10.1016/S0006-3495(96)79438-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart O. S., Breed J., Smith G. R., Sansom M. S. A novel method for structure-based prediction of ion channel conductance properties. Biophys J. 1997 Mar;72(3):1109–1126. doi: 10.1016/S0006-3495(97)78760-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart O. S., Goodfellow J. M., Wallace B. A. The pore dimensions of gramicidin A. Biophys J. 1993 Dec;65(6):2455–2460. doi: 10.1016/S0006-3495(93)81293-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. O., Peersen O. B. Solid-state NMR approaches for studying membrane protein structure. Annu Rev Biophys Biomol Struct. 1992;21:25–47. doi: 10.1146/annurev.bb.21.060192.000325. [DOI] [PubMed] [Google Scholar]
- Taglialatela M., Champagne M. S., Drewe J. A., Brown A. M. Comparison of H5, S6, and H5-S6 exchanges on pore properties of voltage-dependent K+ channels. J Biol Chem. 1994 May 13;269(19):13867–13873. [PubMed] [Google Scholar]
- Taglialatela M., Drewe J. A., Brown A. M. Barium blockade of a clonal potassium channel and its regulation by a critical pore residue. Mol Pharmacol. 1993 Jul;44(1):180–190. [PubMed] [Google Scholar]
- Taglialatela M., Drewe J. A., Kirsch G. E., De Biasi M., Hartmann H. A., Brown A. M. Regulation of K+/Rb+ selectivity and internal TEA blockade by mutations at a single site in K+ pores. Pflugers Arch. 1993 Apr;423(1-2):104–112. doi: 10.1007/BF00374967. [DOI] [PubMed] [Google Scholar]
- Taglialatela M., Kirsch G. E., VanDongen A. M., Drewe J. A., Hartmann H. A., Joho R. H., Stefani E., Brown A. M. Gating currents from a delayed rectifier K+ channel with altered pore structure and function. Biophys J. 1992 Apr;62(1):34–36. doi: 10.1016/S0006-3495(92)81770-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempel B. L., Papazian D. M., Schwarz T. L., Jan Y. N., Jan L. Y. Sequence of a probable potassium channel component encoded at Shaker locus of Drosophila. Science. 1987 Aug 14;237(4816):770–775. doi: 10.1126/science.2441471. [DOI] [PubMed] [Google Scholar]
- Tsukihara T., Aoyama H., Yamashita E., Tomizaki T., Yamaguchi H., Shinzawa-Itoh K., Nakashima R., Yaono R., Yoshikawa S. The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 A. Science. 1996 May 24;272(5265):1136–1144. doi: 10.1126/science.272.5265.1136. [DOI] [PubMed] [Google Scholar]
- Unwin N. Acetylcholine receptor channel imaged in the open state. Nature. 1995 Jan 5;373(6509):37–43. doi: 10.1038/373037a0. [DOI] [PubMed] [Google Scholar]
- Unwin N. Nicotinic acetylcholine receptor at 9 A resolution. J Mol Biol. 1993 Feb 20;229(4):1101–1124. doi: 10.1006/jmbi.1993.1107. [DOI] [PubMed] [Google Scholar]
- Weiss M. S., Kreusch A., Schiltz E., Nestel U., Welte W., Weckesser J., Schulz G. E. The structure of porin from Rhodobacter capsulatus at 1.8 A resolution. FEBS Lett. 1991 Mar 25;280(2):379–382. doi: 10.1016/0014-5793(91)80336-2. [DOI] [PubMed] [Google Scholar]
- Wu J., Kaback H. R. A general method for determining helix packing in membrane proteins in situ: helices I and II are close to helix VII in the lactose permease of Escherichia coli. Proc Natl Acad Sci U S A. 1996 Dec 10;93(25):14498–14502. doi: 10.1073/pnas.93.25.14498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Voss J., Hubbell W. L., Kaback H. R. Site-directed spin labeling and chemical crosslinking demonstrate that helix V is close to helices VII and VIII in the lactose permease of Escherichia coli. Proc Natl Acad Sci U S A. 1996 Sep 17;93(19):10123–10127. doi: 10.1073/pnas.93.19.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Jan Y. N., Jan L. Y. Determination of the subunit stoichiometry of an inwardly rectifying potassium channel. Neuron. 1995 Dec;15(6):1441–1447. doi: 10.1016/0896-6273(95)90021-7. [DOI] [PubMed] [Google Scholar]
- Yellen G., Jurman M. E., Abramson T., MacKinnon R. Mutations affecting internal TEA blockade identify the probable pore-forming region of a K+ channel. Science. 1991 Feb 22;251(4996):939–942. doi: 10.1126/science.2000494. [DOI] [PubMed] [Google Scholar]
- Yellen G., Sodickson D., Chen T. Y., Jurman M. E. An engineered cysteine in the external mouth of a K+ channel allows inactivation to be modulated by metal binding. Biophys J. 1994 Apr;66(4):1068–1075. doi: 10.1016/S0006-3495(94)80888-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yool A. J., Schwarz T. L. Alteration of ionic selectivity of a K+ channel by mutation of the H5 region. Nature. 1991 Feb 21;349(6311):700–704. doi: 10.1038/349700a0. [DOI] [PubMed] [Google Scholar]
- Yool A. J., Schwarz T. L. Interactions of the H5 pore region and hydroxylamine with N-type inactivation in the Shaker K+ channel. Biophys J. 1995 Feb;68(2):448–458. doi: 10.1016/S0006-3495(95)80206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Kono M., McKee T. D., Oprian D. D. A general method for mapping tertiary contacts between amino acid residues in membrane-embedded proteins. Biochemistry. 1995 Nov 21;34(46):14963–14969. doi: 10.1021/bi00046a002. [DOI] [PubMed] [Google Scholar]