Abstract
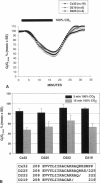
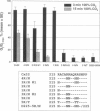
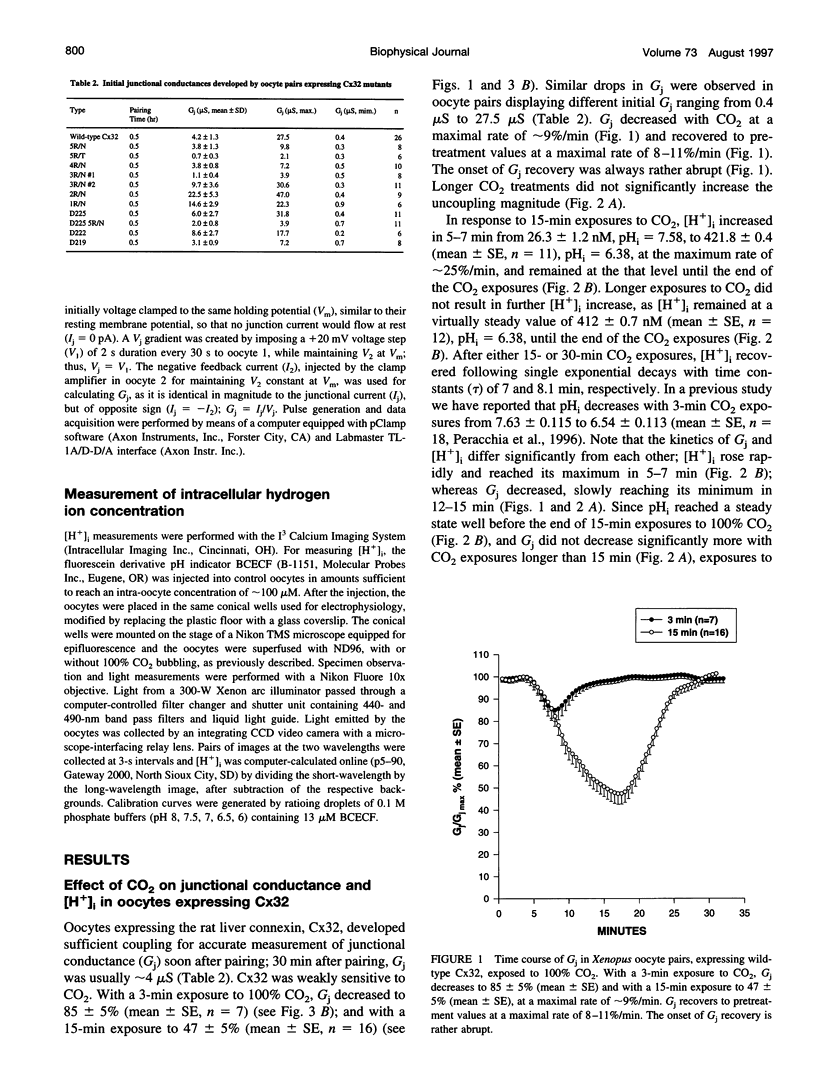
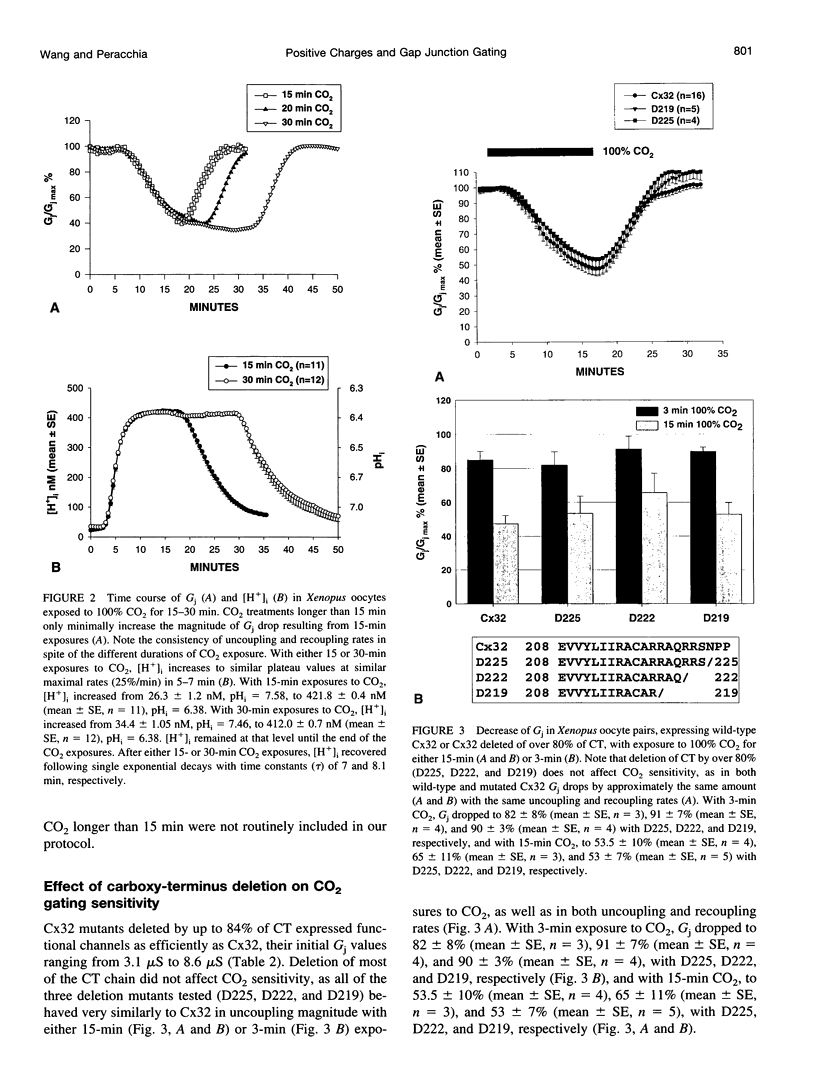
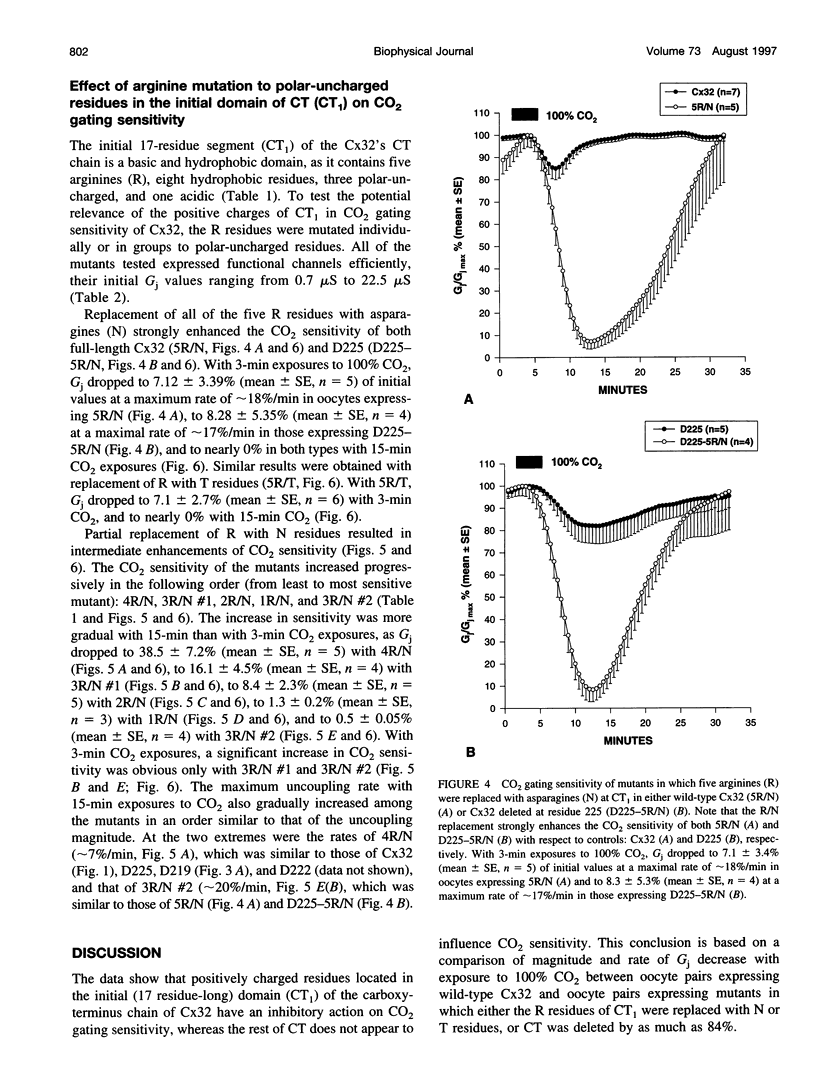
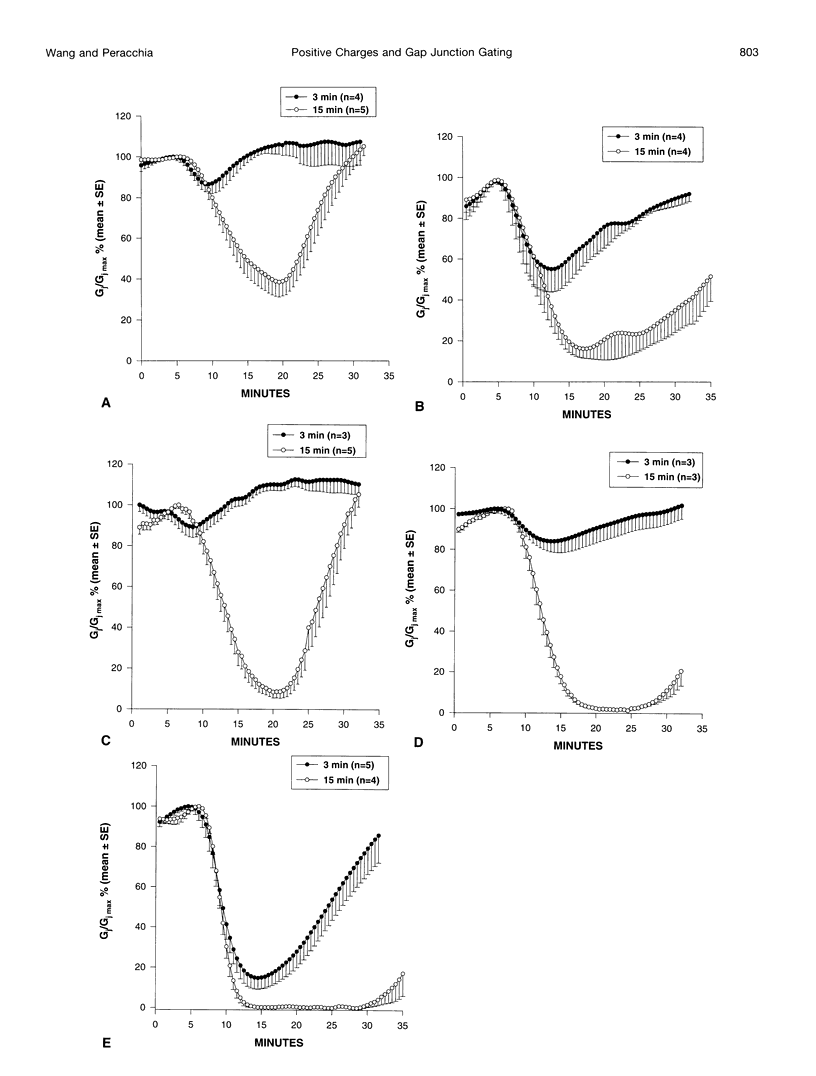
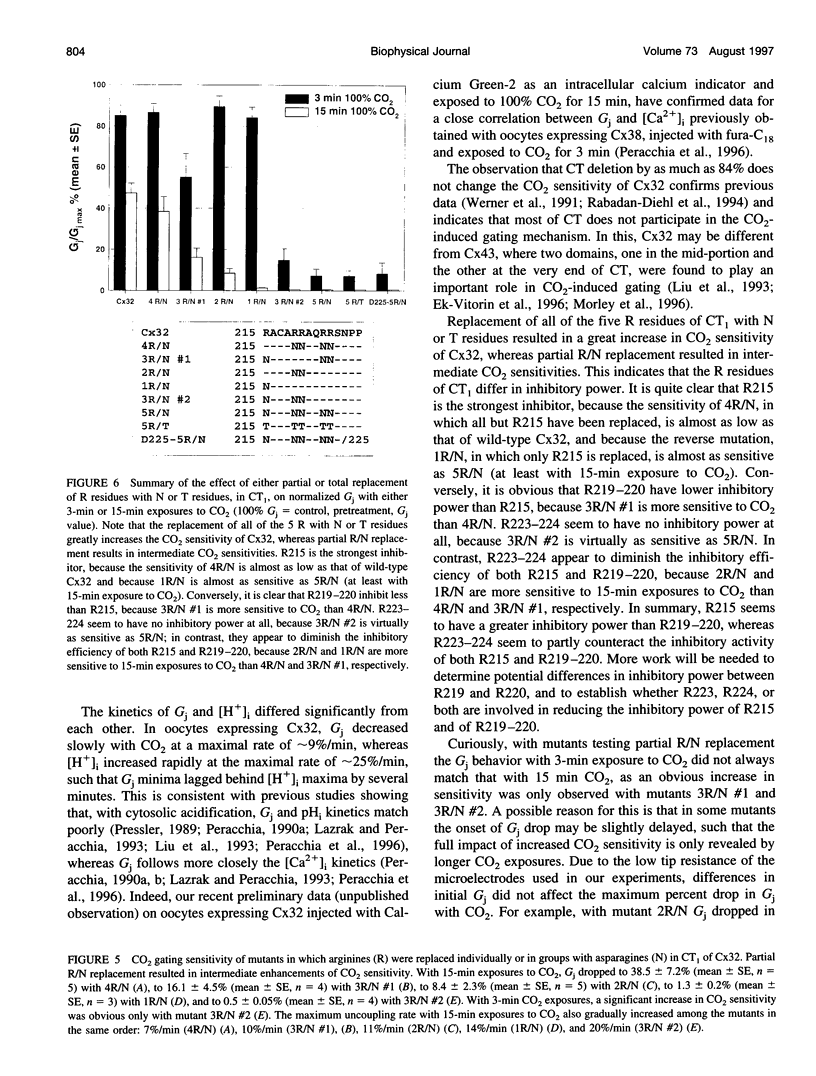
Gap junction channels close with CO2 exposure. To determine whether the carboxy-terminus (CT) of connexin32 (Cx32) participates in gating, the CO2 sensitivity of channels made of Cx32 or Cx32 mutants was studied by double voltage clamp. In Xanopus laevis oocytes expressing Cx32, junctional conductance (Gj) dropped to 85% and 47% of controls with 3- and 15-min CO2 exposures, respectively. In response to the 15-min exposure to CO2, pHi dropped to approximately 6.4 in 5-7 min and did not decrease further, even with 30-min exposures. CT deletion by 84% did not affect CO2 sensitivity, but replacement of five arginines (R215, R219, R220, R223, and R224) with asparagines (N) or threonines at the beginning of CT (CT1) in Cx32 or Cx32 deleted beyond residue 225 greatly enhanced CO2 sensitivity (with 3-min CO2 Gj dropped to approximately 8%). Partial R/N replacement resulted in intermediate CO2 sensitivity enhancement. R215 is a stronger inhibitor than R219-220, whereas R223-224 may diminish the inhibitory efficiency of R215 and R219-220. Therefore, positive charges of CT1 reduce the CO2 sensitivity of Cx32, whereas the rest (> 80%) of CT seems to play no role in CO2-induced gating. The role of presumed electrostatic interactions among Cx32 domains in CO2-induced gating is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M. Time course of TEA(+)-induced anomalous rectification in squid giant axons. J Gen Physiol. 1966 Nov;50(2):491–503. doi: 10.1085/jgp.50.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrio L. C., Suchyna T., Bargiello T., Xu L. X., Roginski R. S., Bennett M. V., Nicholson B. J. Gap junctions formed by connexins 26 and 32 alone and in combination are differently affected by applied voltage. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8410–8414. doi: 10.1073/pnas.88.19.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzone R., White T. W., Paul D. L. Connections with connexins: the molecular basis of direct intercellular signaling. Eur J Biochem. 1996 May 15;238(1):1–27. doi: 10.1111/j.1432-1033.1996.0001q.x. [DOI] [PubMed] [Google Scholar]
- Ek-Vitorín J. F., Calero G., Morley G. E., Coombs W., Taffet S. M., Delmar M. PH regulation of connexin43: molecular analysis of the gating particle. Biophys J. 1996 Sep;71(3):1273–1284. doi: 10.1016/S0006-3495(96)79328-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazrak A., Peracchia C. Gap junction gating sensitivity to physiological internal calcium regardless of pH in Novikoff hepatoma cells. Biophys J. 1993 Nov;65(5):2002–2012. doi: 10.1016/S0006-3495(93)81242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Taffet S., Stoner L., Delmar M., Vallano M. L., Jalife J. A structural basis for the unequal sensitivity of the major cardiac and liver gap junctions to intracellular acidification: the carboxyl tail length. Biophys J. 1993 May;64(5):1422–1433. doi: 10.1016/S0006-3495(93)81508-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley G. E., Taffet S. M., Delmar M. Intramolecular interactions mediate pH regulation of connexin43 channels. Biophys J. 1996 Mar;70(3):1294–1302. doi: 10.1016/S0006-3495(96)79686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul D. L. Molecular cloning of cDNA for rat liver gap junction protein. J Cell Biol. 1986 Jul;103(1):123–134. doi: 10.1083/jcb.103.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peracchia C. Effects of caffeine and ryanodine on low pHi-induced changes in gap junction conductance and calcium concentration in crayfish septate axons. J Membr Biol. 1990 Jul;117(1):79–89. doi: 10.1007/BF01871567. [DOI] [PubMed] [Google Scholar]
- Peracchia C. Increase in gap junction resistance with acidification in crayfish septate axons is closely related to changes in intracellular calcium but not hydrogen ion concentration. J Membr Biol. 1990 Jan;113(1):75–92. doi: 10.1007/BF01869608. [DOI] [PubMed] [Google Scholar]
- Peracchia C., Wang X., Li L., Peracchia L. L. Inhibition of calmodulin expression prevents low-pH-induced gap junction uncoupling in Xenopus oocytes. Pflugers Arch. 1996 Jan;431(3):379–387. doi: 10.1007/BF02207275. [DOI] [PubMed] [Google Scholar]
- Pressler M. L. Intracellular pH and cell-to-cell transmission in sheep Purkinje fibers. Biophys J. 1989 Jan;55(1):53–65. doi: 10.1016/S0006-3495(89)82780-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabadan-Diehl C., Dahl G., Werner R. A connexin-32 mutation associated with Charcot-Marie-Tooth disease does not affect channel formation in oocytes. FEBS Lett. 1994 Aug 29;351(1):90–94. doi: 10.1016/0014-5793(94)00819-1. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Burt J. M. Structure-activity relations of the cardiac gap junction channel. Am J Physiol. 1990 Feb;258(2 Pt 1):C195–C205. doi: 10.1152/ajpcell.1990.258.2.C195. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Harris A. L., Bennett M. V. Equilibrium properties of a voltage-dependent junctional conductance. J Gen Physiol. 1981 Jan;77(1):77–93. doi: 10.1085/jgp.77.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turin L., Warner A. Carbon dioxide reversibly abolishes ionic communication between cells of early amphibian embryo. Nature. 1977 Nov 3;270(5632):56–57. doi: 10.1038/270056a0. [DOI] [PubMed] [Google Scholar]
- Wang X. G., Peracchia C. Connexin 32/38 chimeras suggest a role for the second half of inner loop in gap junction gating by low pH. Am J Physiol. 1996 Nov;271(5 Pt 1):C1743–C1749. doi: 10.1152/ajpcell.1996.271.5.C1743. [DOI] [PubMed] [Google Scholar]
- Wang X., Li L., Peracchia L. L., Peracchia C. Chimeric evidence for a role of the connexin cytoplasmic loop in gap junction channel gating. Pflugers Arch. 1996 Apr;431(6):844–852. doi: 10.1007/s004240050076. [DOI] [PubMed] [Google Scholar]
- Werner R., Levine E., Rabadan-Diehl C., Dahl G. Gating properties of connexin32 cell-cell channels and their mutants expressed in Xenopus oocytes. Proc Biol Sci. 1991 Jan 22;243(1306):5–11. doi: 10.1098/rspb.1991.0002. [DOI] [PubMed] [Google Scholar]
- Zagotta W. N., Hoshi T., Aldrich R. W. Restoration of inactivation in mutants of Shaker potassium channels by a peptide derived from ShB. Science. 1990 Oct 26;250(4980):568–571. doi: 10.1126/science.2122520. [DOI] [PubMed] [Google Scholar]