Abstract
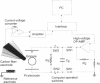
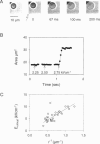
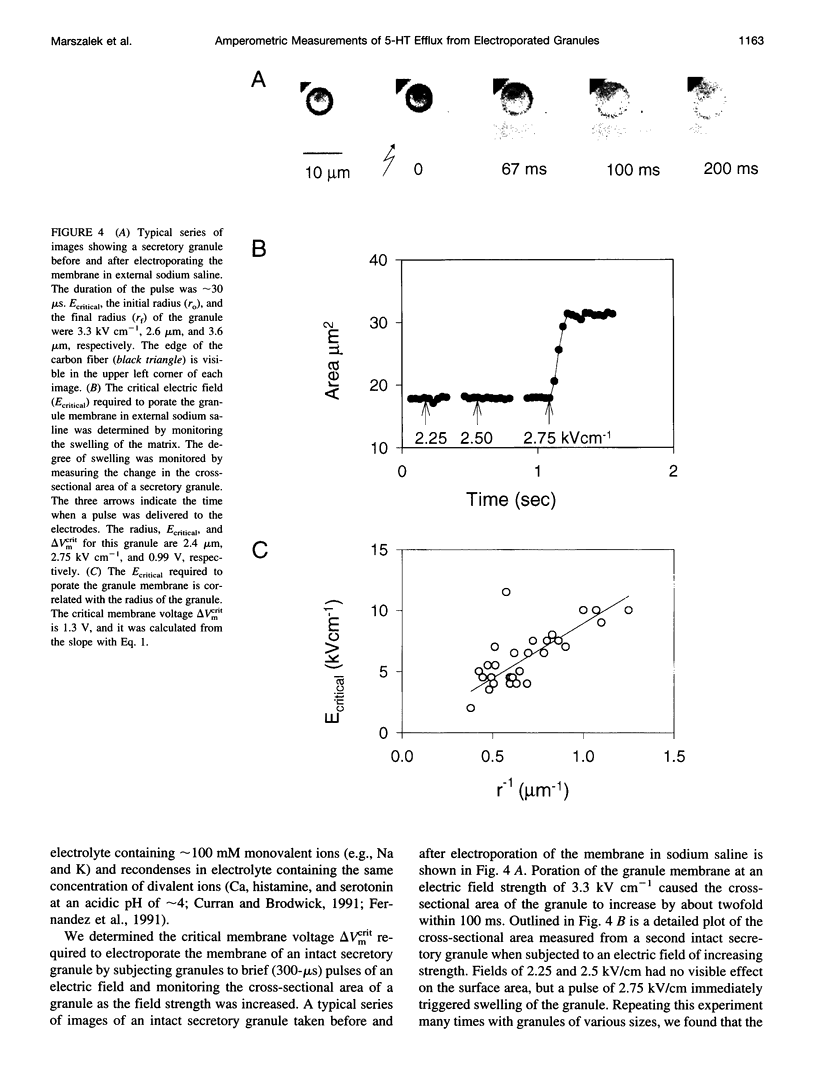
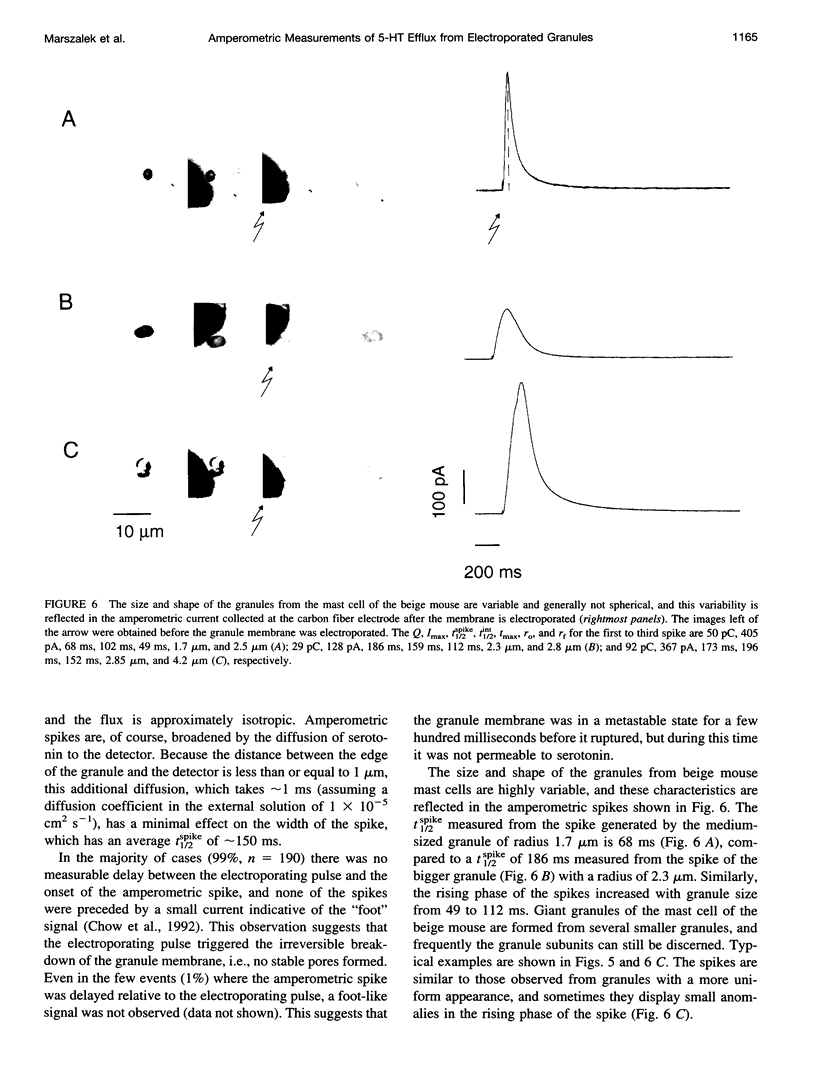
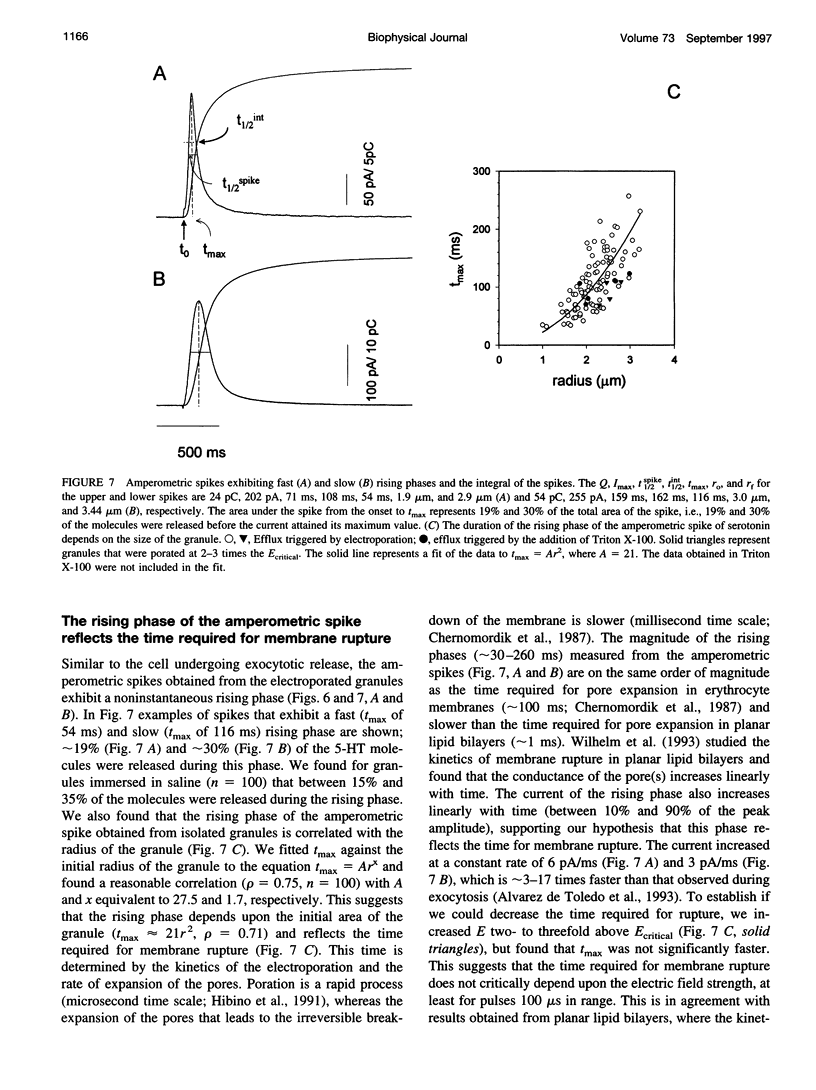
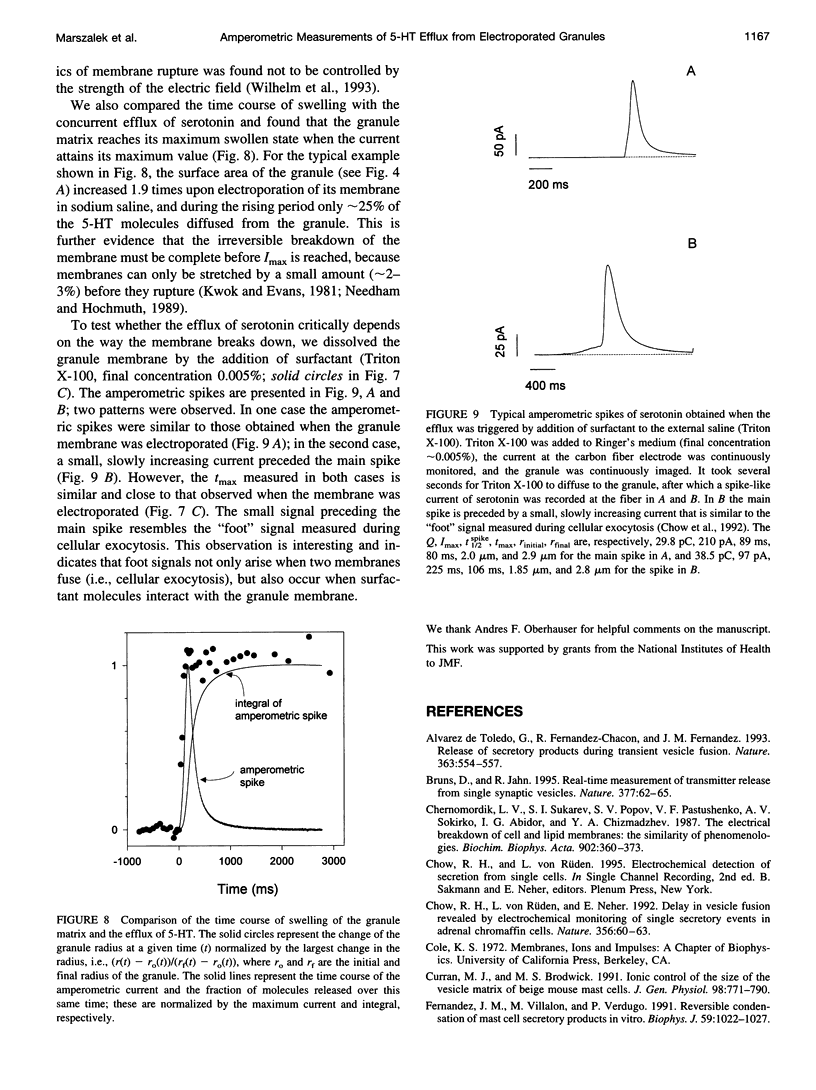
We developed a method for measuring the efflux of 5-hydroxytryptamine (5-HT, serotonin) from isolated intact granules of the mast cell of the beige mouse. This method combines electroporation of the vesicle membrane with amperometric detection of 5-HT. A single secretory granule is placed between two platinum electrodes (distance approximately 100 microm) and positioned adjacent (<1 microm) to a carbon fiber microelectrode. A short (approximately 30 micros) high-intensity voltage pulse (electric field of approximately 5 kV/cm) is delivered to the electrodes to trigger the mechanical breakdown of the granule membrane, which activates the release of 5-HT. We observed concurrent swelling of the granule matrix with the oxidation of 5-HT at the carbon fiber electrode (overpotential + 650 mV). Similar to the release of secretory products during exocytosis, the oxidation current exhibits a spike-like time course with a noninstantaneous rising phase (time between onset of current and maximum flux, t(max)) with approximately 25% of the molecules released during this period. When the current reaches its maximum, the granule matrix attains its maximum swollen state. We found that the rising phase depends on the initial cross-sectional area of the granule (t(max) approximately 21r2) and reflects the time required for membrane rupture. The average t(1/2)spike of the amperometric spikes was found to be approximately 150 ms, which is 3-7 times faster than the t(1/2) measured during cellular exocytosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez de Toledo G., Fernández-Chacón R., Fernández J. M. Release of secretory products during transient vesicle fusion. Nature. 1993 Jun 10;363(6429):554–558. doi: 10.1038/363554a0. [DOI] [PubMed] [Google Scholar]
- Bruns D., Jahn R. Real-time measurement of transmitter release from single synaptic vesicles. Nature. 1995 Sep 7;377(6544):62–65. doi: 10.1038/377062a0. [DOI] [PubMed] [Google Scholar]
- Chernomordik L. V., Sukharev S. I., Popov S. V., Pastushenko V. F., Sokirko A. V., Abidor I. G., Chizmadzhev Y. A. The electrical breakdown of cell and lipid membranes: the similarity of phenomenologies. Biochim Biophys Acta. 1987 Sep 3;902(3):360–373. doi: 10.1016/0005-2736(87)90204-5. [DOI] [PubMed] [Google Scholar]
- Chow R. H., von Rüden L., Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992 Mar 5;356(6364):60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- Curran M. J., Brodwick M. S. Ionic control of the size of the vesicle matrix of beige mouse mast cells. J Gen Physiol. 1991 Oct;98(4):771–790. doi: 10.1085/jgp.98.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez J. M., Villalón M., Verdugo P. Reversible condensation of mast cell secretory products in vitro. Biophys J. 1991 May;59(5):1022–1027. doi: 10.1016/S0006-3495(91)82317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino M., Shigemori M., Itoh H., Nagayama K., Kinosita K., Jr Membrane conductance of an electroporated cell analyzed by submicrosecond imaging of transmembrane potential. Biophys J. 1991 Jan;59(1):209–220. doi: 10.1016/S0006-3495(91)82212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski J. A., Schroeder T. J., Ciolkowski E. L., Wightman R. M. Temporal characteristics of quantal secretion of catecholamines from adrenal medullary cells. J Biol Chem. 1993 Jul 15;268(20):14694–14700. [PubMed] [Google Scholar]
- Kawagoe K. T., Zimmerman J. B., Wightman R. M. Principles of voltammetry and microelectrode surface states. J Neurosci Methods. 1993 Jul;48(3):225–240. doi: 10.1016/0165-0270(93)90094-8. [DOI] [PubMed] [Google Scholar]
- Kwok R., Evans E. Thermoelasticity of large lecithin bilayer vesicles. Biophys J. 1981 Sep;35(3):637–652. doi: 10.1016/S0006-3495(81)84817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leszczyszyn D. J., Jankowski J. A., Viveros O. H., Diliberto E. J., Jr, Near J. A., Wightman R. M. Nicotinic receptor-mediated catecholamine secretion from individual chromaffin cells. Chemical evidence for exocytosis. J Biol Chem. 1990 Sep 5;265(25):14736–14737. [PubMed] [Google Scholar]
- Marszalek P. E., Farrell B., Verdugo P., Fernandez J. M. Kinetics of release of serotonin from isolated secretory granules. II. Ion exchange determines the diffusivity of serotonin. Biophys J. 1997 Sep;73(3):1169–1183. doi: 10.1016/S0006-3495(97)78149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanavati C., Fernandez J. M. The secretory granule matrix: a fast-acting smart polymer. Science. 1993 Feb 12;259(5097):963–965. doi: 10.1126/science.8438154. [DOI] [PubMed] [Google Scholar]
- Needham D., Hochmuth R. M. Electro-mechanical permeabilization of lipid vesicles. Role of membrane tension and compressibility. Biophys J. 1989 May;55(5):1001–1009. doi: 10.1016/S0006-3495(89)82898-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhauser A. F., Fernandez J. M. Patch clamp studies of single intact secretory granules. Biophys J. 1993 Nov;65(5):1844–1852. doi: 10.1016/S0006-3495(93)81246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihel K., Travis E. R., Borges R., Wightman R. M. Exocytotic release from individual granules exhibits similar properties at mast and chromaffin cells. Biophys J. 1996 Sep;71(3):1633–1640. doi: 10.1016/S0006-3495(96)79368-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder T. J., Borges R., Finnegan J. M., Pihel K., Amatore C., Wightman R. M. Temporally resolved, independent stages of individual exocytotic secretion events. Biophys J. 1996 Feb;70(2):1061–1068. doi: 10.1016/S0006-3495(96)79652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruce A. E., Breckenridge L. J., Lee A. K., Almers W. Properties of the fusion pore that forms during exocytosis of a mast cell secretory vesicle. Neuron. 1990 May;4(5):643–654. doi: 10.1016/0896-6273(90)90192-i. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y. Electroporation of cell membranes. Biophys J. 1991 Aug;60(2):297–306. doi: 10.1016/S0006-3495(91)82054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ureña J., Fernández-Chacón R., Benot A. R., Alvarez de Toledo G. A., López-Barneo J. Hypoxia induces voltage-dependent Ca2+ entry and quantal dopamine secretion in carotid body glomus cells. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10208–10211. doi: 10.1073/pnas.91.21.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman R. M., Schroeder T. J., Finnegan J. M., Ciolkowski E. L., Pihel K. Time course of release of catecholamines from individual vesicles during exocytosis at adrenal medullary cells. Biophys J. 1995 Jan;68(1):383–390. doi: 10.1016/S0006-3495(95)80199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm C., Winterhalter M., Zimmermann U., Benz R. Kinetics of pore size during irreversible electrical breakdown of lipid bilayer membranes. Biophys J. 1993 Jan;64(1):121–128. doi: 10.1016/S0006-3495(93)81346-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Misler S., Chow R. H. Rapid fluctuations in transmitter release from single vesicles in bovine adrenal chromaffin cells. Biophys J. 1996 Mar;70(3):1543–1552. doi: 10.1016/S0006-3495(96)79718-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann U. Electrical breakdown, electropermeabilization and electrofusion. Rev Physiol Biochem Pharmacol. 1986;105:176–256. [PubMed] [Google Scholar]