Abstract
2H Double quantum-filtered (DQF) NMR spectroscopy of deuterated water is sensitive to the presence of order in biological systems. This is because the only nuclei that are detected are those with residual quadrupolar interactions due to their anisotropic motion. In the present study, samples of aorta, coronary and carotid arteries, and vena cava were studied in parallel by 2H DQF NMR and by light microscopy. The average quadrupolar splitting, calculated from the NMR data, varies considerably among the different blood vessels, with high reproducibility for each type of vessel. Polarization microscopy examinations using collagen-specific staining with picrosirius red, have shown a variety of color profiles for the different blood vessels. These reflect different physical modes of aggregation (packing and thickness) of collagen fibers. A correlation was found between the NMR parameters and the color profiles of the picrosirius red-stained sections. Treating the blood vessels with 90% formic acid resulted in the elimination of the 2H DQF NMR signal. Histological analysis demonstrated a complete degradation of collagen and muscle, whereas the elastin filaments were preserved. Evidence is given that the 2H DQF NMR signal is dominated by the contribution of water molecules interacting with the collagen fibers.
Full text
PDF

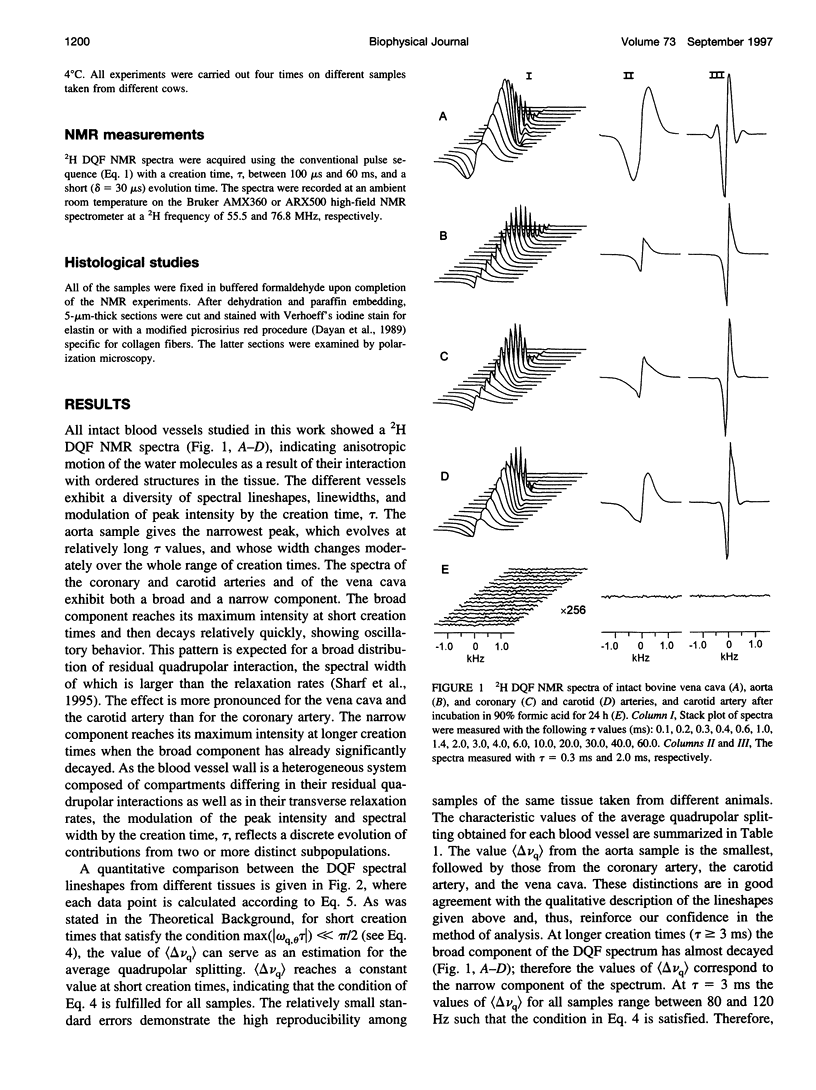
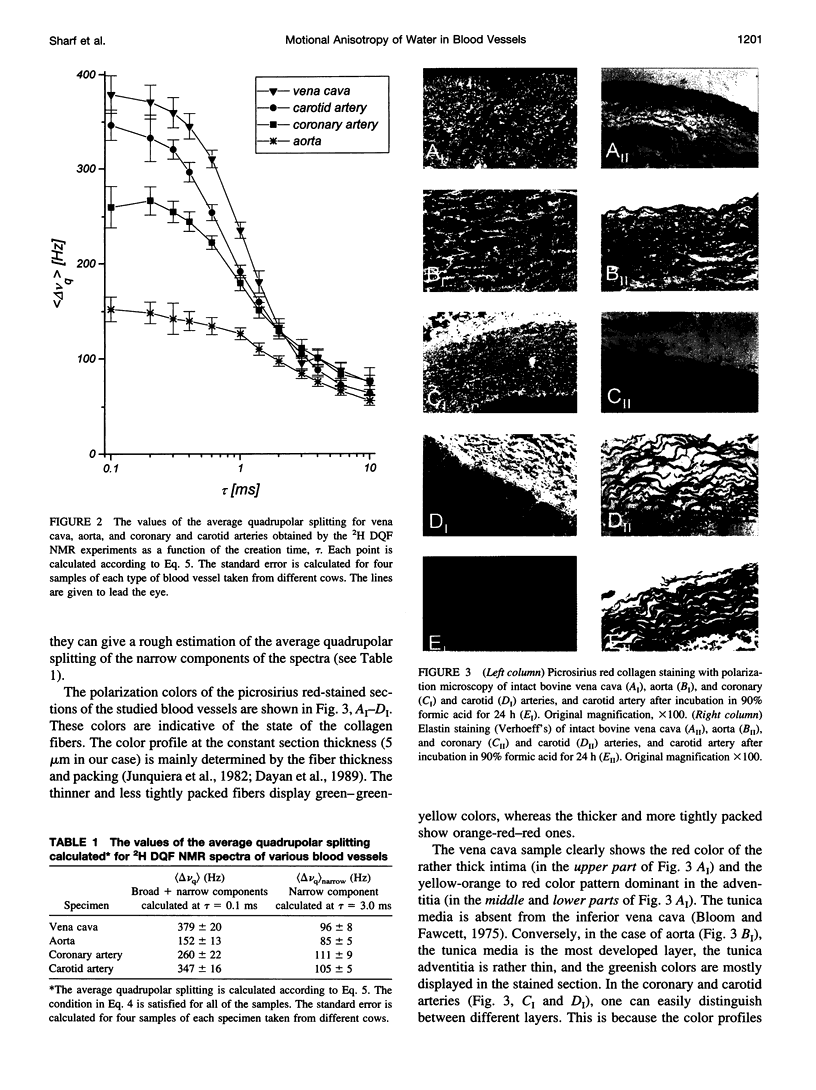



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assaf Y., Navon G., Cohen Y. In vivo observation of anisotropic motion of brain water using 2H double quantum filtered NMR spectroscopy. Magn Reson Med. 1997 Feb;37(2):197–203. doi: 10.1002/mrm.1910370210. [DOI] [PubMed] [Google Scholar]
- Constantine V. S., Mowry R. W. Selective staining of human dermal collagen. II. The use of picrosirius red F3BA with polarization microscopy. J Invest Dermatol. 1968 May;50(5):419–423. doi: 10.1038/jid.1968.68. [DOI] [PubMed] [Google Scholar]
- Dayan D., Hiss Y., Hirshberg A., Bubis J. J., Wolman M. Are the polarization colors of picrosirius red-stained collagen determined only by the diameter of the fibers? Histochemistry. 1989;93(1):27–29. doi: 10.1007/BF00266843. [DOI] [PubMed] [Google Scholar]
- Dayan D., Waner T., Tal H., Nyska A. Polarization microscopy of picrosirius red-stained collagen from oxodipine-induced hyperplastic gingiva of beagle dogs. Int J Exp Pathol. 1993 Jun;74(3):225–228. [PMC free article] [PubMed] [Google Scholar]
- Eliav U., Navon G. Analysis of double-quantum-filtered NMR spectra of 23Na in biological tissues. J Magn Reson B. 1994 Jan;103(1):19–29. doi: 10.1006/jmrb.1994.1003. [DOI] [PubMed] [Google Scholar]
- Hirschberg A., Buchner A., Dayan D. The central odontogenic fibroma and the hyperplastic dental follicle: study with Picrosirius red and polarizing microscopy. J Oral Pathol Med. 1996 Mar;25(3):125–127. doi: 10.1111/j.1600-0714.1996.tb00206.x. [DOI] [PubMed] [Google Scholar]
- Jackson D. S., Cleary E. G. The determination of collagen and elastin. Methods Biochem Anal. 1967;15:25–76. doi: 10.1002/9780470110331.ch2. [DOI] [PubMed] [Google Scholar]
- Junqueira L. C., Bignolas G., Brentani R. R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979 Jul;11(4):447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- Junqueira L. C., Montes G. S., Sanchez E. M. The influence of tissue section thickness on the study of collagen by the Picrosirius-polarization method. Histochemistry. 1982;74(1):153–156. doi: 10.1007/BF00495061. [DOI] [PubMed] [Google Scholar]
- Knubovets T., Shinar H., Eliav U., Navon G. A 23Na multiple-quantum-filtered NMR study of the effect of the cytoskeleton conformation on the anisotropic motion of sodium ions in red blood cells. J Magn Reson B. 1996 Jan;110(1):16–25. doi: 10.1006/jmrb.1996.0003. [DOI] [PubMed] [Google Scholar]
- Kreis R., Boesch C. Liquid-crystal-like structures of human muscle demonstrated by in vivo observation of direct dipolar coupling in localized proton magnetic resonance spectroscopy. J Magn Reson B. 1994 Jun;104(2):189–192. doi: 10.1006/jmrb.1994.1075. [DOI] [PubMed] [Google Scholar]
- Kreis R., Koster M., Kamber M., Hoppeler H., Boesch C. Peak assignment in localized 1H MR spectra of human muscle based on oral creatine supplementation. Magn Reson Med. 1997 Feb;37(2):159–163. doi: 10.1002/mrm.1910370202. [DOI] [PubMed] [Google Scholar]
- Reddy R., Bolinger L., Shinnar M., Noyszewski E., Leigh J. S. Detection of residual quadrupolar interaction in human skeletal muscle and brain in vivo via multiple quantum filtered sodium NMR spectra. Magn Reson Med. 1995 Jan;33(1):134–139. doi: 10.1002/mrm.1910330121. [DOI] [PubMed] [Google Scholar]
- Sharf Y., Akselrod S., Navon G. Measurement of strain exerted on blood vessel walls by double-quantum-filtered 2H NMR. Magn Reson Med. 1997 Jan;37(1):69–75. doi: 10.1002/mrm.1910370111. [DOI] [PubMed] [Google Scholar]
- Shinar H., Knubovets T., Eliav U., Navon G. Sodium interaction with ordered structures in mammalian red blood cells detected by Na-23 double quantum NMR. Biophys J. 1993 Apr;64(4):1273–1279. doi: 10.1016/S0006-3495(93)81492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinée P., Meurer B., Constantinesco A., Kohlberger B., Hauenstein K. H., Laubenberger J. In vitro proton NMR study of collagen in human aortic wall. Magn Reson Med. 1993 Mar;29(3):292–295. doi: 10.1002/mrm.1910290303. [DOI] [PubMed] [Google Scholar]
- Yan Y., Winograd E., Viel A., Cronin T., Harrison S. C., Branton D. Crystal structure of the repetitive segments of spectrin. Science. 1993 Dec 24;262(5142):2027–2030. doi: 10.1126/science.8266097. [DOI] [PubMed] [Google Scholar]



