Abstract
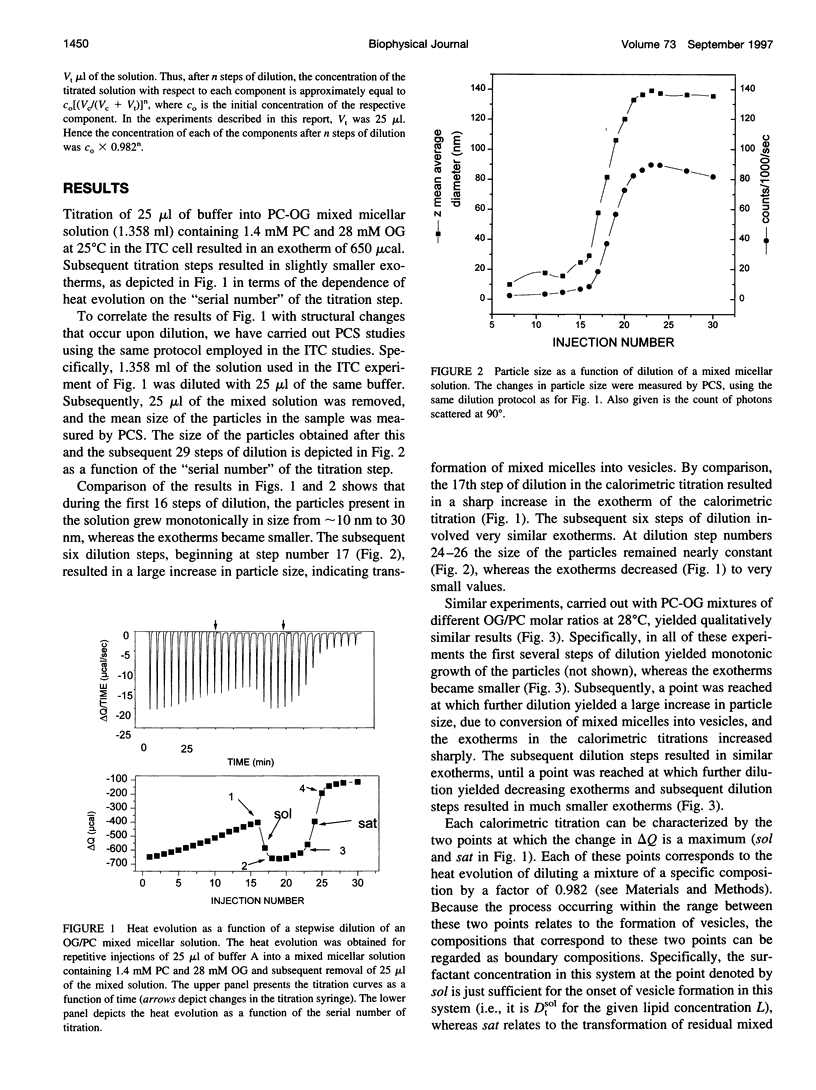
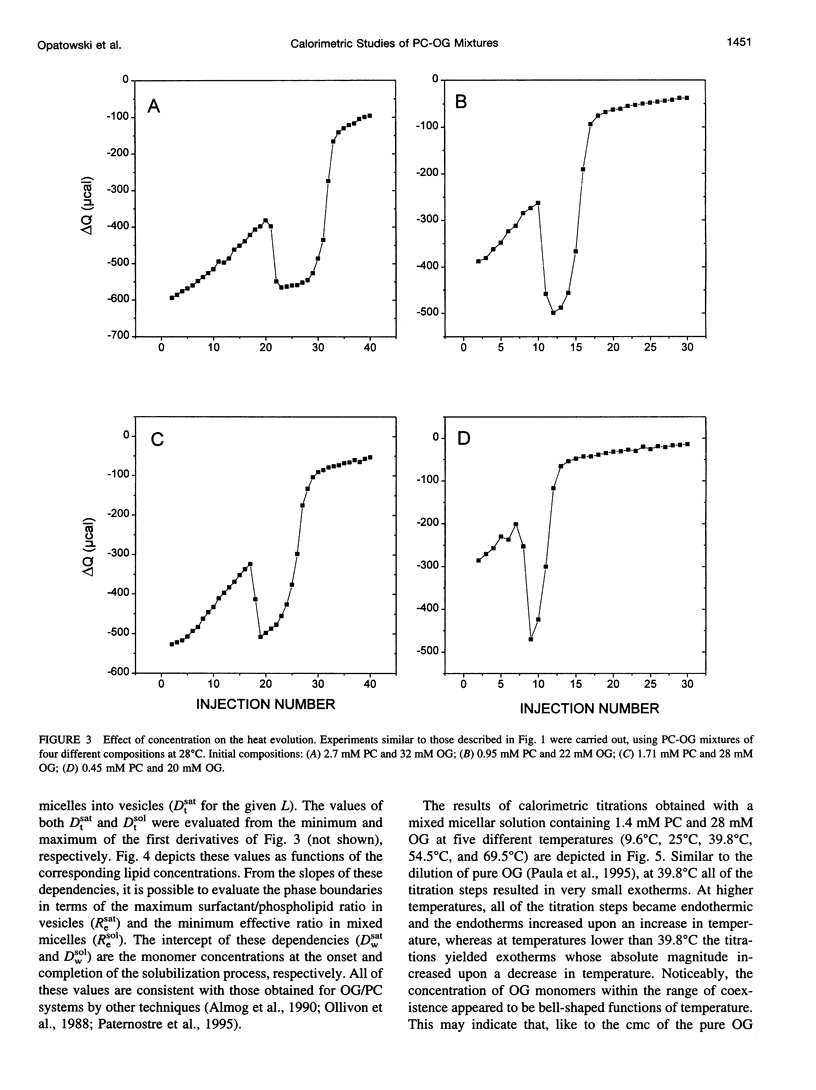
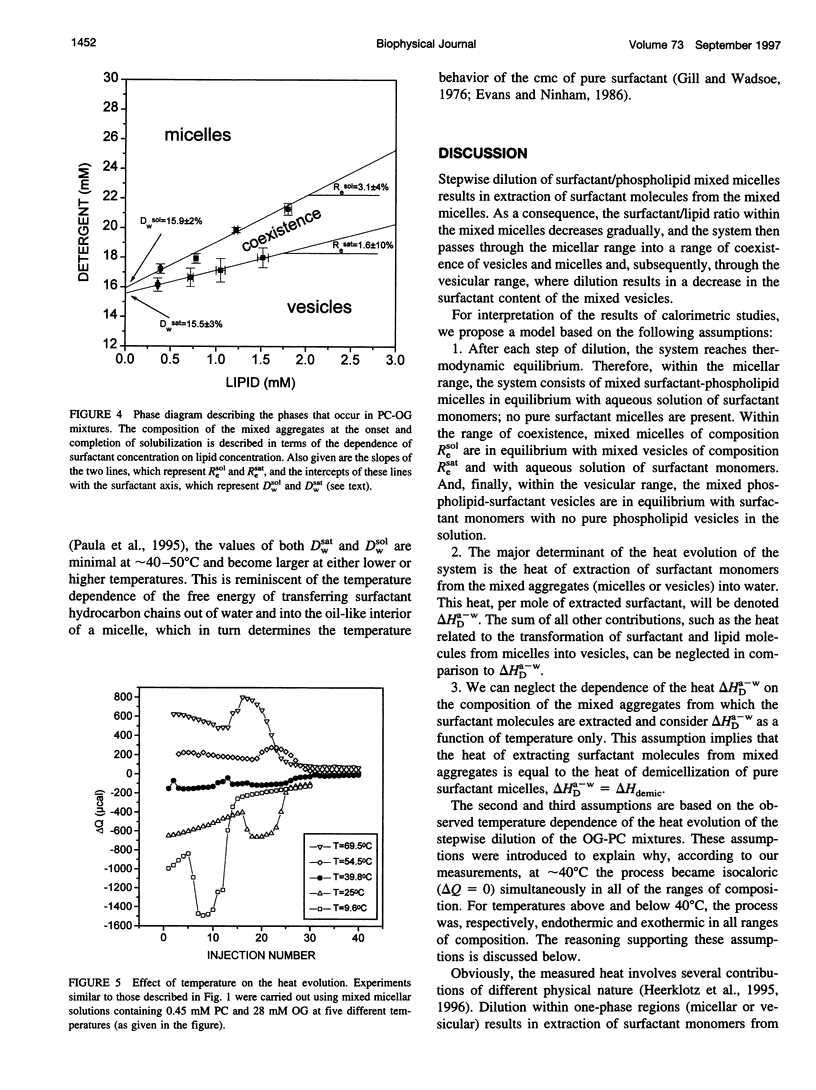
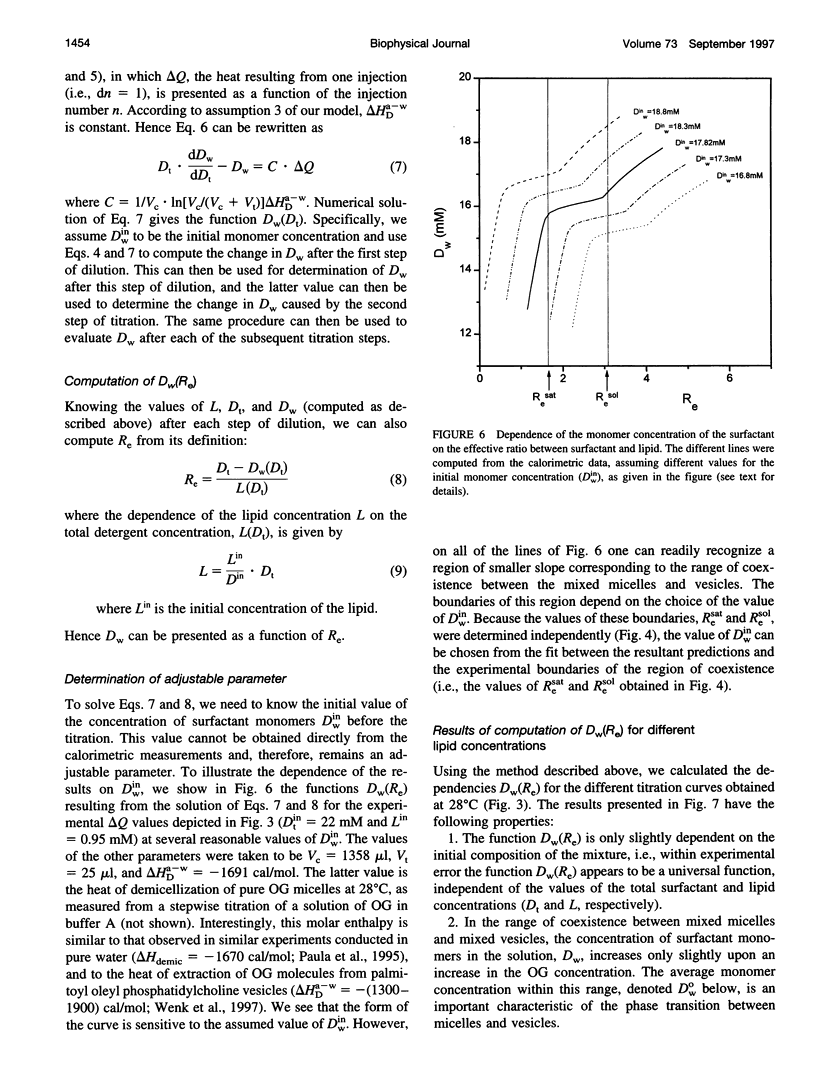
Stepwise dilution of lipid-surfactant mixed micelles first results in extraction of surfactant from the mixed micelles into the aqueous medium. Subsequently mixed micelles transform into vesicles, within a range of compositions that corresponds to equilibrium coexistence between these two types of aggregates. Further dilution results in extraction of surfactant from the resultant mixed vesicles. In the present study, we have investigated the heat evolution of these processes, as they occur in mixed systems composed of egg phosphatidylcholine (PC) and the nonionic surfactant octylglucoside (OG). A combined use of isothermal titration calorimetry (ITC) and photon correlation spectroscopy (PCS), capable of monitoring phase transformations, revealed that 1) The sum of all of the studied processes (i.e., extraction of OG from mixed micelles and vesicles and the phase transformation) is isocaloric at approximately 40 degrees C throughout the whole dilution. At lower temperatures, all of the dilution steps are exothermic, whereas at higher temperatures all of them are endothermic. 2) At all temperatures, the absolute value of the heat associated with each dilution step within the range of coexistence of micelles and vesicles is almost constant and larger than in either the micellar or the vesicular range. We give an interpretation of these calorimetric data in terms of the relationship between the composition of the mixed aggregates Re and the aqueous concentration of surfactant monomers Dw. Assuming that the main contribution to the heat evolution is due to extraction of surfactant from mixed aggregates to the aqueous solution, we deduce the relationship Dw(Re) characterizing the system over the whole range of compositions. We find that, in accord with thermodynamic expectations, Dw is almost constant throughout the range of coexistence of mixed micelles and vesicles.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almog S., Kushnir T., Nir S., Lichtenberg D. Kinetic and structural aspects of reconstitution of phosphatidylcholine vesicles by dilution of phosphatidylcholine-sodium cholate mixed micelles. Biochemistry. 1986 May 6;25(9):2597–2605. doi: 10.1021/bi00357a048. [DOI] [PubMed] [Google Scholar]
- Almog S., Litman B. J., Wimley W., Cohen J., Wachtel E. J., Barenholz Y., Ben-Shaul A., Lichtenberg D. States of aggregation and phase transformations in mixtures of phosphatidylcholine and octyl glucoside. Biochemistry. 1990 May 15;29(19):4582–4592. doi: 10.1021/bi00471a012. [DOI] [PubMed] [Google Scholar]
- Chiarantini L., Serafini G., Stocchi V., Magnani M. Preparation and characterization of monoclonal antibodies to human hexokinase type I. Mol Cell Biochem. 1990 Sep 21;97(2):145–151. doi: 10.1007/BF00221056. [DOI] [PubMed] [Google Scholar]
- Eidelman O., Blumenthal R., Walter A. Composition of octyl glucoside-phosphatidylcholine mixed micelles. Biochemistry. 1988 Apr 19;27(8):2839–2846. doi: 10.1021/bi00408a027. [DOI] [PubMed] [Google Scholar]
- Emerson M. F., Holtzer A. The hydrophobic bond in micellar systems. Effects of various additives on the stability of micelles of sodium dodecyl sulfate and of n-dodecyltrimethylammonium bromide. J Phys Chem. 1967 Sep;71(10):3320–3330. doi: 10.1021/j100869a031. [DOI] [PubMed] [Google Scholar]
- Epand R. M., Epand R. F. Calorimetric detection of curvature strain in phospholipid bilayers. Biophys J. 1994 May;66(5):1450–1456. doi: 10.1016/S0006-3495(94)80935-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S. J., Wadsö I. An equation of state describing hydrophobic interactions. Proc Natl Acad Sci U S A. 1976 Sep;73(9):2955–2958. doi: 10.1073/pnas.73.9.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goñi F. M., Urbaneja M. A., Arrondo J. L., Alonso A., Durrani A. A., Chapman D. The interaction of phosphatidylcholine bilayers with Triton X-100. Eur J Biochem. 1986 Nov 3;160(3):659–665. doi: 10.1111/j.1432-1033.1986.tb10088.x. [DOI] [PubMed] [Google Scholar]
- Gruner S. M., Parsegian V. A., Rand R. P. Directly measured deformation energy of phospholipid HII hexagonal phases. Faraday Discuss Chem Soc. 1986;(81):29–37. doi: 10.1039/dc9868100029. [DOI] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Israelachvili J. N., Marcelja S., Horn R. G. Physical principles of membrane organization. Q Rev Biophys. 1980 May;13(2):121–200. doi: 10.1017/s0033583500001645. [DOI] [PubMed] [Google Scholar]
- Jackson M. L., Schmidt C. F., Lichtenberg D., Litman B. J., Albert A. D. Solubilization of phosphatidylcholine bilayers by octyl glucoside. Biochemistry. 1982 Sep 14;21(19):4576–4582. doi: 10.1021/bi00262a010. [DOI] [PubMed] [Google Scholar]
- Kresheck G. C., Nimsgern R. A. Unusual enthalpy changes which accompany the titration of dimyristoylphosphatidylcholine vesicles with Triton X-100. Chem Phys Lipids. 1983 Jul;33(1):55–65. doi: 10.1016/0009-3084(83)90008-7. [DOI] [PubMed] [Google Scholar]
- Lichtenberg D. Characterization of the solubilization of lipid bilayers by surfactants. Biochim Biophys Acta. 1985 Dec 19;821(3):470–478. doi: 10.1016/0005-2736(85)90052-5. [DOI] [PubMed] [Google Scholar]
- Lichtenberg D., Robson R. J., Dennis E. A. Solubilization of phospholipids by detergents. Structural and kinetic aspects. Biochim Biophys Acta. 1983 May 24;737(2):285–304. doi: 10.1016/0304-4157(83)90004-7. [DOI] [PubMed] [Google Scholar]
- Ollivon M., Eidelman O., Blumenthal R., Walter A. Micelle-vesicle transition of egg phosphatidylcholine and octyl glucoside. Biochemistry. 1988 Mar 8;27(5):1695–1703. doi: 10.1021/bi00405a047. [DOI] [PubMed] [Google Scholar]
- Paternostre M. T., Roux M., Rigaud J. L. Mechanisms of membrane protein insertion into liposomes during reconstitution procedures involving the use of detergents. 1. Solubilization of large unilamellar liposomes (prepared by reverse-phase evaporation) by triton X-100, octyl glucoside, and sodium cholate. Biochemistry. 1988 Apr 19;27(8):2668–2677. doi: 10.1021/bi00408a006. [DOI] [PubMed] [Google Scholar]
- Paternostre M., Meyer O., Grabielle-Madelmont C., Lesieur S., Ghanam M., Ollivon M. Partition coefficient of a surfactant between aggregates and solution: application to the micelle-vesicle transition of egg phosphatidylcholine and octyl beta-D-glucopyranoside. Biophys J. 1995 Dec;69(6):2476–2488. doi: 10.1016/S0006-3495(95)80118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno M. Partition behavior of a nonionic detergent, octyl glucoside, between membrane and water phases, and its effect on membrane permeability. Biochemistry. 1989 Jun 27;28(13):5631–5634. doi: 10.1021/bi00439a044. [DOI] [PubMed] [Google Scholar]
- Vinson P. K., Talmon Y., Walter A. Vesicle-micelle transition of phosphatidylcholine and octyl glucoside elucidated by cryo-transmission electron microscopy. Biophys J. 1989 Oct;56(4):669–681. doi: 10.1016/S0006-3495(89)82714-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter A., Vinson P. K., Kaplun A., Talmon Y. Intermediate structures in the cholate-phosphatidylcholine vesicle-micelle transition. Biophys J. 1991 Dec;60(6):1315–1325. doi: 10.1016/S0006-3495(91)82169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk M. R., Alt T., Seelig A., Seelig J. Octyl-beta-D-glucopyranoside partitioning into lipid bilayers: thermodynamics of binding and structural changes of the bilayer. Biophys J. 1997 Apr;72(4):1719–1731. doi: 10.1016/S0006-3495(97)78818-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerer R. O., Jr, Lindenbaum S. Enthalpy of bile salt-lecithin mixed micelle formation. J Pharm Sci. 1979 May;68(5):581–585. doi: 10.1002/jps.2600680517. [DOI] [PubMed] [Google Scholar]
- da Graça Miguel M., Eidelman O., Ollivon M., Walter A. Temperature dependence of the vesicle-micelle transition of egg phosphatidylcholine and octyl glucoside. Biochemistry. 1989 Oct 31;28(22):8921–8928. doi: 10.1021/bi00448a035. [DOI] [PubMed] [Google Scholar]


