Abstract
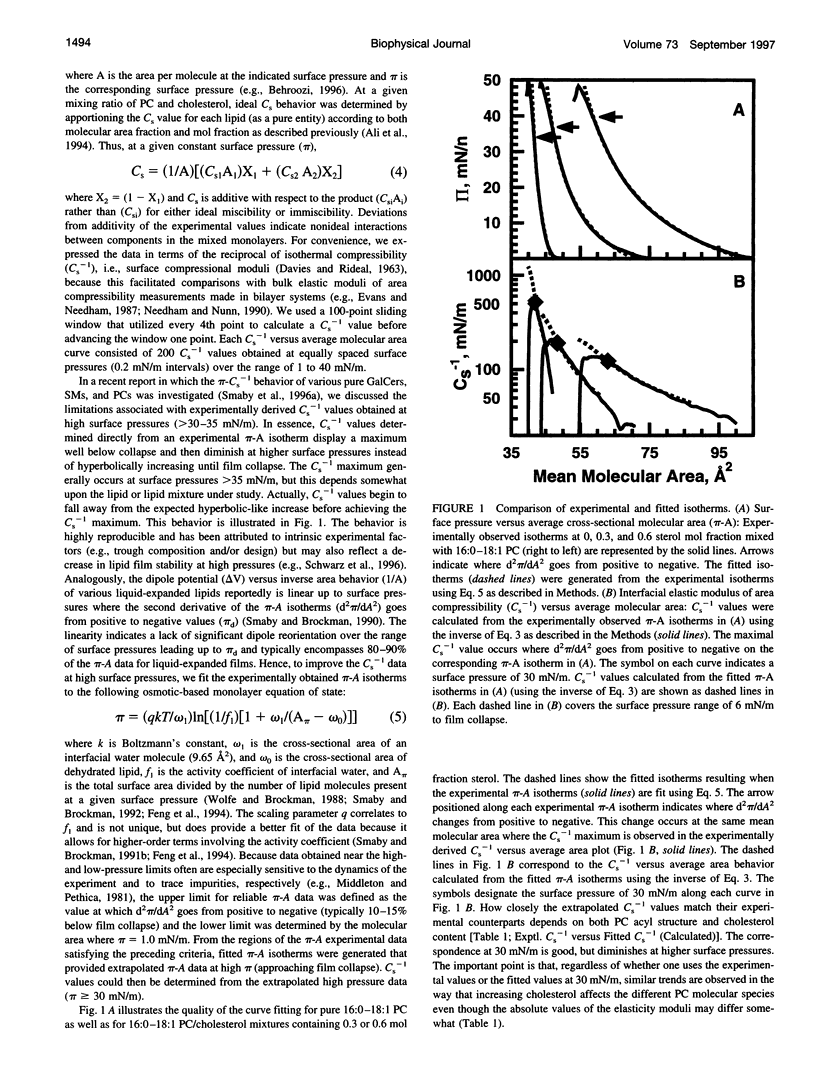
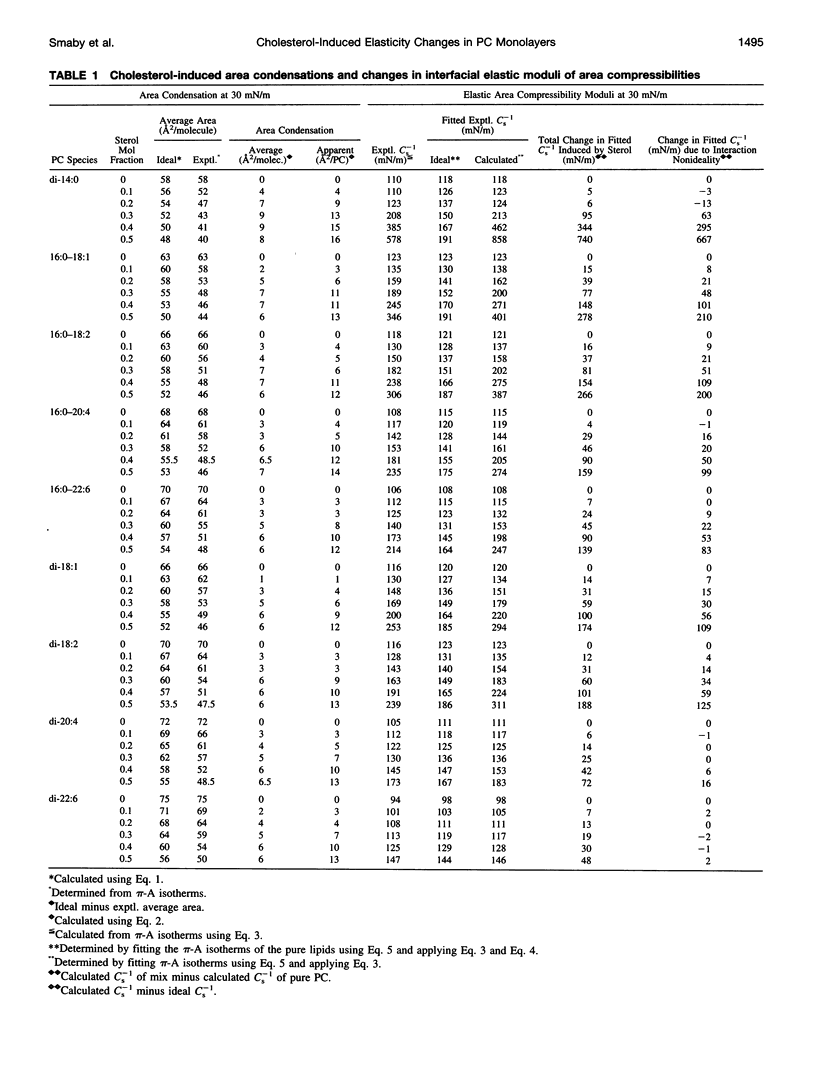
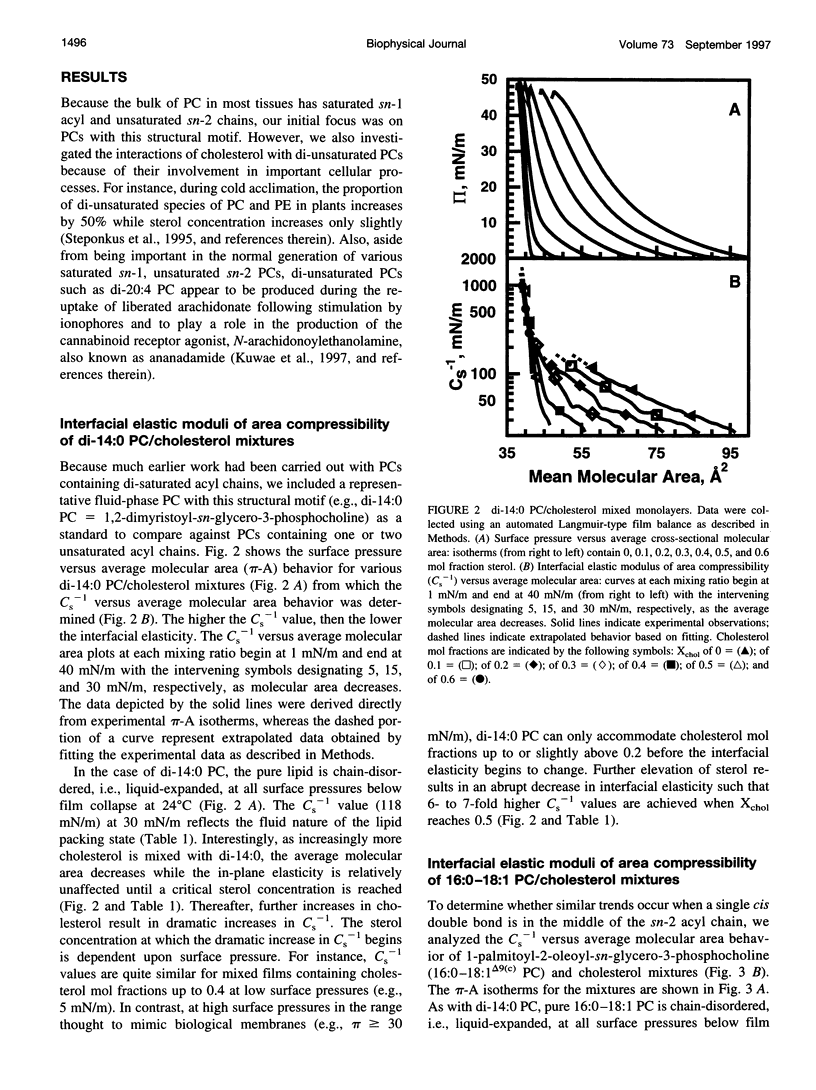
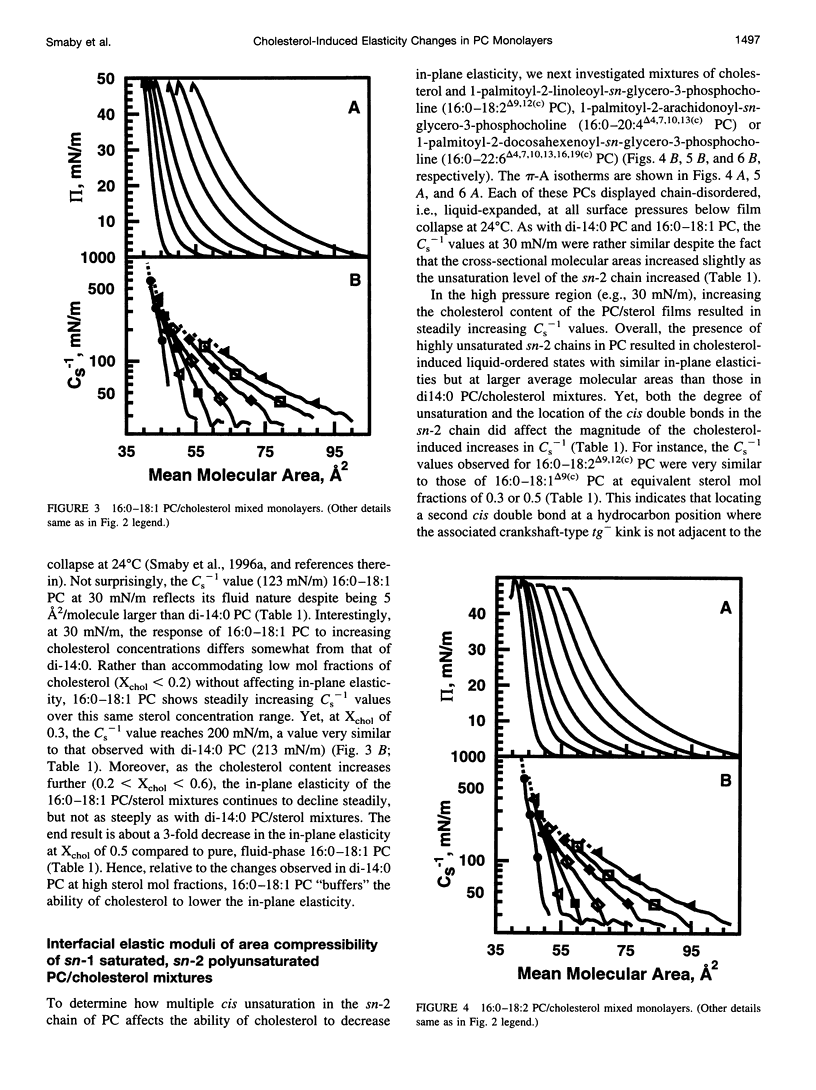
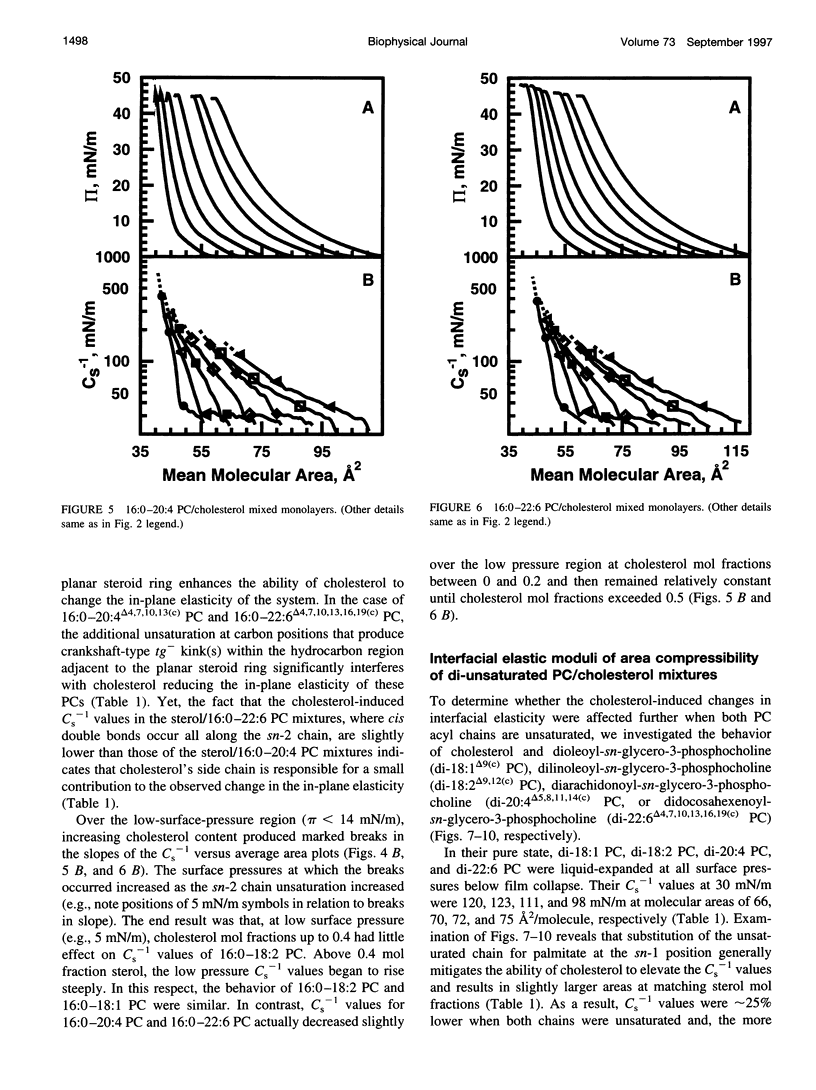
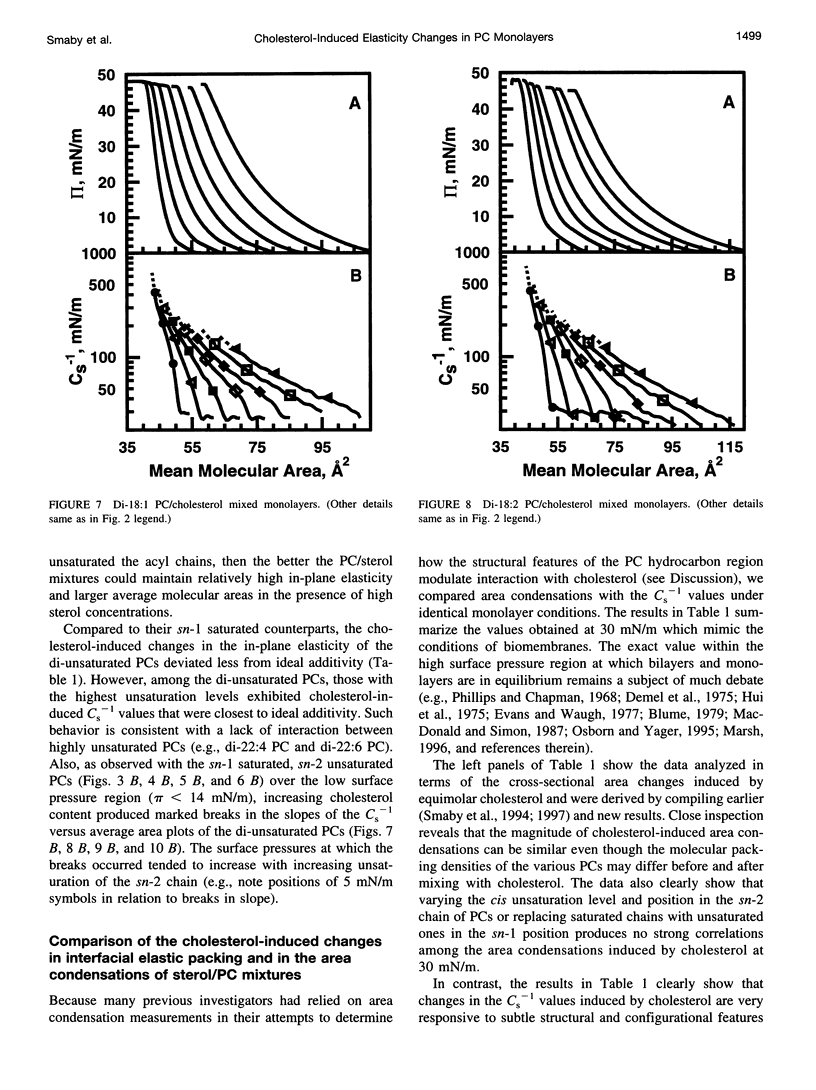
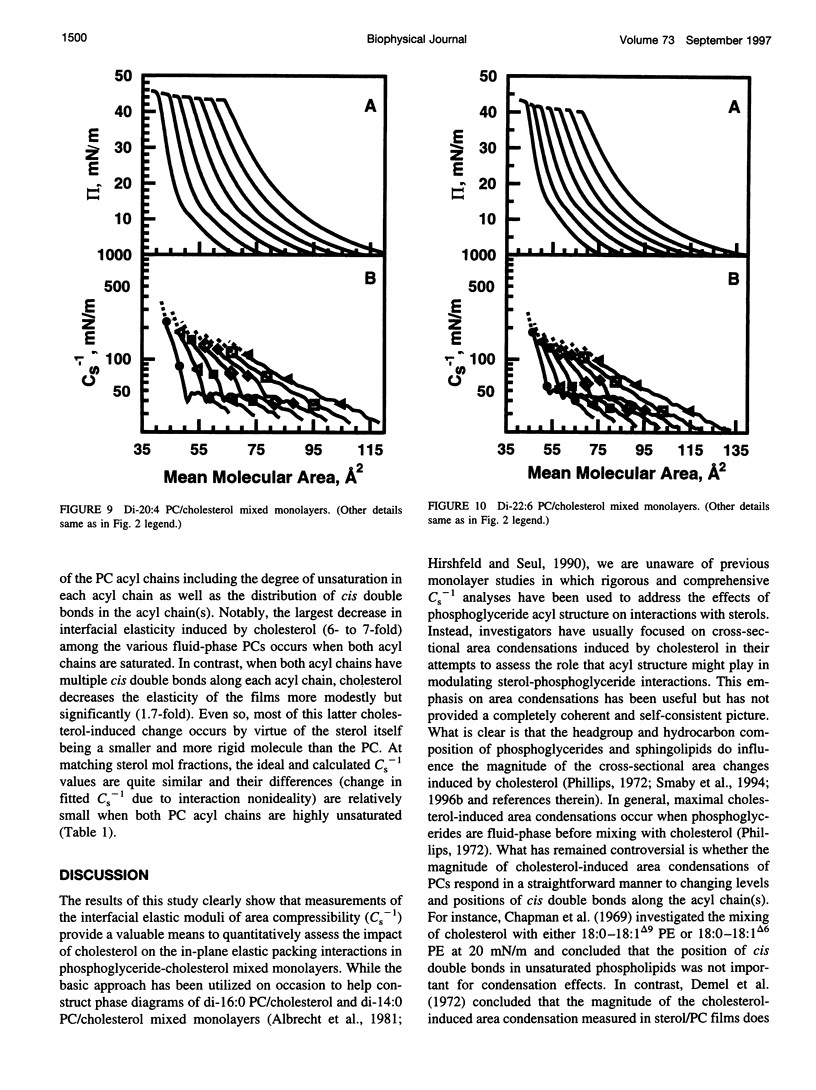
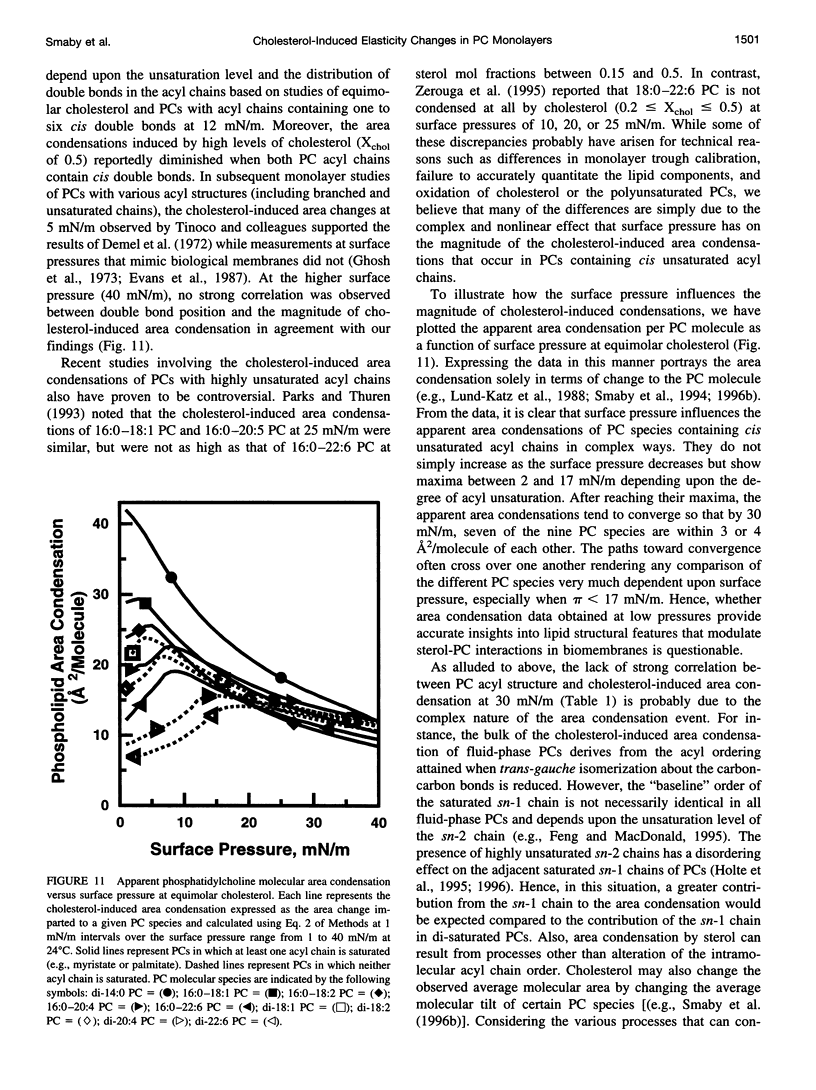
The effect of cholesterol on the interfacial elastic packing interactions of various molecular species of phosphatidylcholines (PCs) has been investigated by using a Langmuir-type film balance and analyzing the elastic area compressibility moduli (Cs(-1)) as a function of average cross-sectional molecular area. Emphasis was on the high surface pressure regions (pi > or = 30 mN/m) which are thought to mimic biomembrane conditions. Increasing levels of cholesterol generally caused the in-plane elasticity of the mixed monolayers to decrease. Yet, the magnitude of the cholesterol-induced changes was markedly dependent upon PC hydrocarbon structure. Among PC species with a saturated sn-1 chain but different sn-2 chain cis unsaturation levels [e.g., myristate (14:0), oleate (18:1delta9(c), linoleate (18:2delta9,12(c), arachidonate (20:4delta5,8,11,14(c), or docosahexenoate (22:6delta4,7,10,13,16,19(c)], the in-plane elasticity moduli of PC species with higher sn-2 unsaturation levels were less affected by high cholesterol mol fractions (e.g., >30 mol %) than were the more saturated PC species. The largest cholesterol-induced decreases in the in-plane elasticity were observed when both chains of PC were saturated (e.g., di-14:0 PC). When both acyl chains were identically unsaturated, the resulting PCs were 20-25% more elastic in the presence of cholesterol than when their sn-1 chains were long and saturated (e.g., palmitate). The mixing of cholesterol with PC was found to diminish the in-plane elasticity of the films beyond what was predicted from the additive behavior of the individual lipid components apportioned by mole and area fraction. Deviations from additivity were greatest for di-14:0 PC and were least for diarachidonoyl PC and didocosahexenoyl PC. In contrast to Cs(-1) analyses, sterol-induced area condensations were relatively unresponsive to subtle structural differences in the PCs at high surface pressures. Cs(-1) versus average area plots also indicated the presence of cholesterol concentration-dependent, low-pressure (<14 mN/m) phase boundaries that became more prominent as PC acyl chain unsaturation increased. Hence, area condensations measured at low surface pressures often do not accurately portray which lipid structural features are important in the lipid-sterol interactions that occur at high membrane-like surface pressures.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S., Smaby J. M., Brockman H. L., Brown R. E. Cholesterol's interfacial interactions with galactosylceramides. Biochemistry. 1994 Mar 15;33(10):2900–2906. doi: 10.1021/bi00176a020. [DOI] [PMC free article] [PubMed] [Google Scholar]
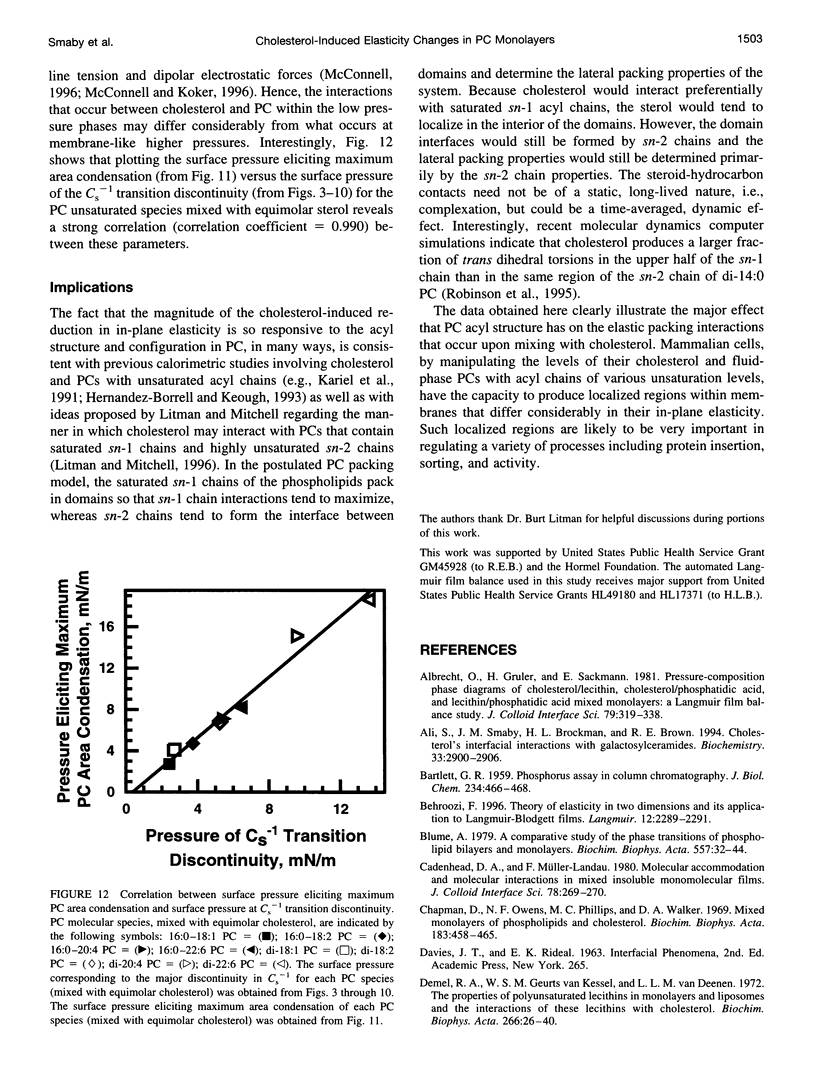
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Blume A. A comparative study of the phase transitions of phospholipid bilayers and monolayers. Biochim Biophys Acta. 1979 Oct 19;557(1):32–44. doi: 10.1016/0005-2736(79)90087-7. [DOI] [PubMed] [Google Scholar]
- Chapman D., Owens N. F., Phillips M. C., Walker D. A. Mixed monolayers of phospholipids and cholesterol. Biochim Biophys Acta. 1969;183(3):458–465. doi: 10.1016/0005-2736(69)90160-6. [DOI] [PubMed] [Google Scholar]
- Demel R. A., Geurts van Kessel W. S., Zwaal R. F., Roelofsen B., van Deenen L. L. Relation between various phospholipase actions on human red cell membranes and the interfacial phospholipid pressure in monolayers. Biochim Biophys Acta. 1975 Sep 16;406(1):97–107. doi: 10.1016/0005-2736(75)90045-0. [DOI] [PubMed] [Google Scholar]
- Demiel R. A., Guerts van Kessel W. S., van Deenen L. L. The properties of polyunsaturated lecithins in monolayers and liposomes and the interactions of these lecithins with cholesterol. Biochim Biophys Acta. 1972 Apr 14;266(1):26–40. doi: 10.1016/0005-2736(72)90116-2. [DOI] [PubMed] [Google Scholar]
- Evans E, Rawicz W. Entropy-driven tension and bending elasticity in condensed-fluid membranes. Phys Rev Lett. 1990 Apr 23;64(17):2094–2097. doi: 10.1103/PhysRevLett.64.2094. [DOI] [PubMed] [Google Scholar]
- Evans R. W., Williams M. A., Tinoco J. Surface areas of 1-palmitoyl phosphatidylcholines and their interactions with cholesterol. Biochem J. 1987 Jul 15;245(2):455–462. doi: 10.1042/bj2450455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S. S., MacDonald R. C. Effects of chain unsaturation on the equation of state for lipid monolayers at the air-water interface. Biophys J. 1995 Aug;69(2):460–469. doi: 10.1016/S0006-3495(95)79919-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D., Williams M. A., Tinoco J. The influence of lecithin structure on their monolayer behavior and interactions with cholesterol. Biochim Biophys Acta. 1973 Jan 26;291(2):351–362. doi: 10.1016/0005-2736(73)90488-4. [DOI] [PubMed] [Google Scholar]
- Hernandez-Borrell J., Keough K. M. Heteroacid phosphatidylcholines with different amounts of unsaturation respond differently to cholesterol. Biochim Biophys Acta. 1993 Dec 12;1153(2):277–282. doi: 10.1016/0005-2736(93)90416-w. [DOI] [PubMed] [Google Scholar]
- Holte L. L., Peter S. A., Sinnwell T. M., Gawrisch K. 2H nuclear magnetic resonance order parameter profiles suggest a change of molecular shape for phosphatidylcholines containing a polyunsaturated acyl chain. Biophys J. 1995 Jun;68(6):2396–2403. doi: 10.1016/S0006-3495(95)80422-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui S. W., Cowden M., Papahadjopoulos D., Parsons D. F. Electron diffraction study of hydrated phospholipid single bilayers. Effects of temperature hydration and surface pressure of the "precursor" monolayer. Biochim Biophys Acta. 1975 Mar 25;382(3):265–275. doi: 10.1016/0005-2736(75)90269-2. [DOI] [PubMed] [Google Scholar]
- Kariel N., Davidson E., Keough K. M. Cholesterol does not remove the gel-liquid crystalline phase transition of phosphatidylcholines containing two polyenoic acyl chains. Biochim Biophys Acta. 1991 Feb 11;1062(1):70–76. doi: 10.1016/0005-2736(91)90336-7. [DOI] [PubMed] [Google Scholar]
- Keough K. M., Giffin B., Matthews P. L. Phosphatidylcholine-cholesterol interactions: bilayers of heteroacid lipids containing linoleate lose calorimetric transitions at low cholesterol concentration. Biochim Biophys Acta. 1989 Jul 24;983(1):51–55. doi: 10.1016/0005-2736(89)90379-9. [DOI] [PubMed] [Google Scholar]
- Kuwae T., Schmid P. C., Schmid H. H. Alterations of fatty acyl turnover in macrophage glycerolipids induced by stimulation. Evidence for enhanced recycling of arachidonic acid. Biochim Biophys Acta. 1997 Jan 7;1344(1):74–86. doi: 10.1016/s0005-2760(96)00135-x. [DOI] [PubMed] [Google Scholar]
- Kwok R., Evans E. Thermoelasticity of large lecithin bilayer vesicles. Biophys J. 1981 Sep;35(3):637–652. doi: 10.1016/S0006-3495(81)84817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund-Katz S., Laboda H. M., McLean L. R., Phillips M. C. Influence of molecular packing and phospholipid type on rates of cholesterol exchange. Biochemistry. 1988 May 3;27(9):3416–3423. doi: 10.1021/bi00409a044. [DOI] [PubMed] [Google Scholar]
- MacDonald R. C., Simon S. A. Lipid monolayer states and their relationships to bilayers. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4089–4093. doi: 10.1073/pnas.84.12.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh D. Lateral pressure in membranes. Biochim Biophys Acta. 1996 Oct 29;1286(3):183–223. doi: 10.1016/s0304-4157(96)00009-3. [DOI] [PubMed] [Google Scholar]
- McConnell H. M. Equilibration rates in lipid monolayers. Proc Natl Acad Sci U S A. 1996 Dec 24;93(26):15001–15003. doi: 10.1073/pnas.93.26.15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. C., Straume M., Litman B. J. Role of sn-1-saturated,sn-2-polyunsaturated phospholipids in control of membrane receptor conformational equilibrium: effects of cholesterol and acyl chain unsaturation on the metarhodopsin I in equilibrium with metarhodopsin II equilibrium. Biochemistry. 1992 Jan 28;31(3):662–670. doi: 10.1021/bi00118a005. [DOI] [PubMed] [Google Scholar]
- Needham D., McIntosh T. J., Evans E. Thermomechanical and transition properties of dimyristoylphosphatidylcholine/cholesterol bilayers. Biochemistry. 1988 Jun 28;27(13):4668–4673. doi: 10.1021/bi00413a013. [DOI] [PubMed] [Google Scholar]
- Needham D., Nunn R. S. Elastic deformation and failure of lipid bilayer membranes containing cholesterol. Biophys J. 1990 Oct;58(4):997–1009. doi: 10.1016/S0006-3495(90)82444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn T. D., Yager P. Modeling success and failure of Langmuir-Blodgett transfer of phospholipid bilayers to silicon dioxide. Biophys J. 1995 Apr;68(4):1364–1373. doi: 10.1016/S0006-3495(95)80309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks J. S., Thuren T. Y. Decreased binding of apoA-I to phosphatidylcholine monolayers containing 22:6 n-3 in the sn-2 position. J Lipid Res. 1993 May;34(5):779–788. [PubMed] [Google Scholar]
- Phillips M. C., Chapman D. Monolayer characteristics of saturated 1,2,-diacyl phosphatidylcholines (lecithins) and phosphatidylethanolamines at the air-water interface. Biochim Biophys Acta. 1968 Nov 5;163(3):301–313. doi: 10.1016/0005-2736(68)90115-6. [DOI] [PubMed] [Google Scholar]
- Robinson A. J., Richards W. G., Thomas P. J., Hann M. M. Behavior of cholesterol and its effect on head group and chain conformations in lipid bilayers: a molecular dynamics study. Biophys J. 1995 Jan;68(1):164–170. doi: 10.1016/S0006-3495(95)80171-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaby J. M., Brockman H. L. A simple method for estimating surfactant impurities in solvents and subphases used for monolayer studies. Chem Phys Lipids. 1991 Jul;58(3):249–252. doi: 10.1016/0009-3084(91)90099-w. [DOI] [PubMed] [Google Scholar]
- Smaby J. M., Brockman H. L., Brown R. E. Cholesterol's interfacial interactions with sphingomyelins and phosphatidylcholines: hydrocarbon chain structure determines the magnitude of condensation. Biochemistry. 1994 Aug 9;33(31):9135–9142. doi: 10.1021/bi00197a016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaby J. M., Brockman H. L. Surface dipole moments of lipids at the argon-water interface. Similarities among glycerol-ester-based lipids. Biophys J. 1990 Jul;58(1):195–204. doi: 10.1016/S0006-3495(90)82365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaby J. M., Kulkarni V. S., Momsen M., Brown R. E. The interfacial elastic packing interactions of galactosylceramides, sphingomyelins, and phosphatidylcholines. Biophys J. 1996 Feb;70(2):868–877. doi: 10.1016/S0006-3495(96)79629-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaby J. M., Momsen M., Kulkarni V. S., Brown R. E. Cholesterol-induced interfacial area condensations of galactosylceramides and sphingomyelins with identical acyl chains. Biochemistry. 1996 May 7;35(18):5696–5704. doi: 10.1021/bi953057k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillwell W., Dallman T., Dumaual A. C., Crump F. T., Jenski L. J. Cholesterol versus alpha-tocopherol: effects on properties of bilayers made from heteroacid phosphatidylcholines. Biochemistry. 1996 Oct 15;35(41):13353–13362. doi: 10.1021/bi961058m. [DOI] [PubMed] [Google Scholar]
- Vanderkooi G. Computation of mixed phosphatidylcholine-cholesterol bilayer structures by energy minimization. Biophys J. 1994 May;66(5):1457–1468. doi: 10.1016/S0006-3495(94)80936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe D. H., Brockman H. L. Regulation of the surface pressure of lipid monolayers and bilayers by the activity of water: derivation and application of an equation of state. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4285–4289. doi: 10.1073/pnas.85.12.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerouga M., Jenski L. J., Stillwell W. Comparison of phosphatidylcholines containing one or two docosahexaenoic acyl chains on properties of phospholipid monolayers and bilayers. Biochim Biophys Acta. 1995 Jun 14;1236(2):266–272. doi: 10.1016/0005-2736(95)00058-b. [DOI] [PubMed] [Google Scholar]


