Abstract
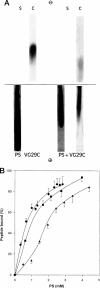
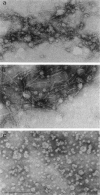
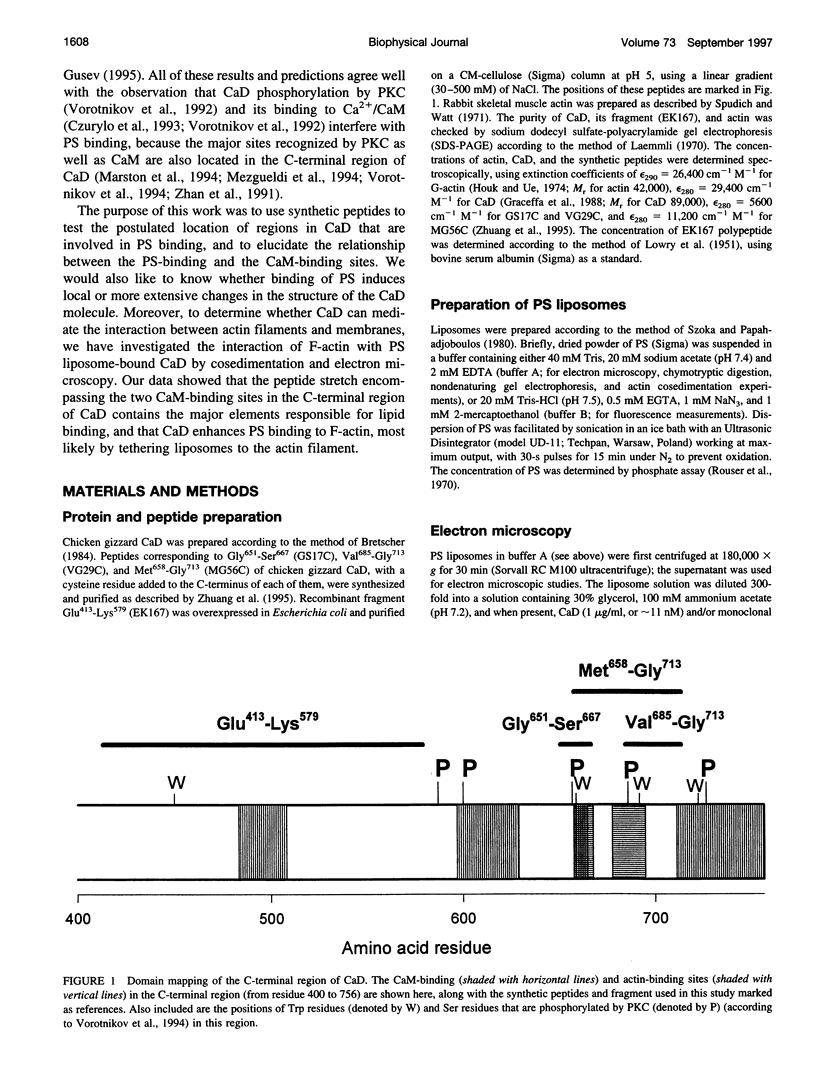
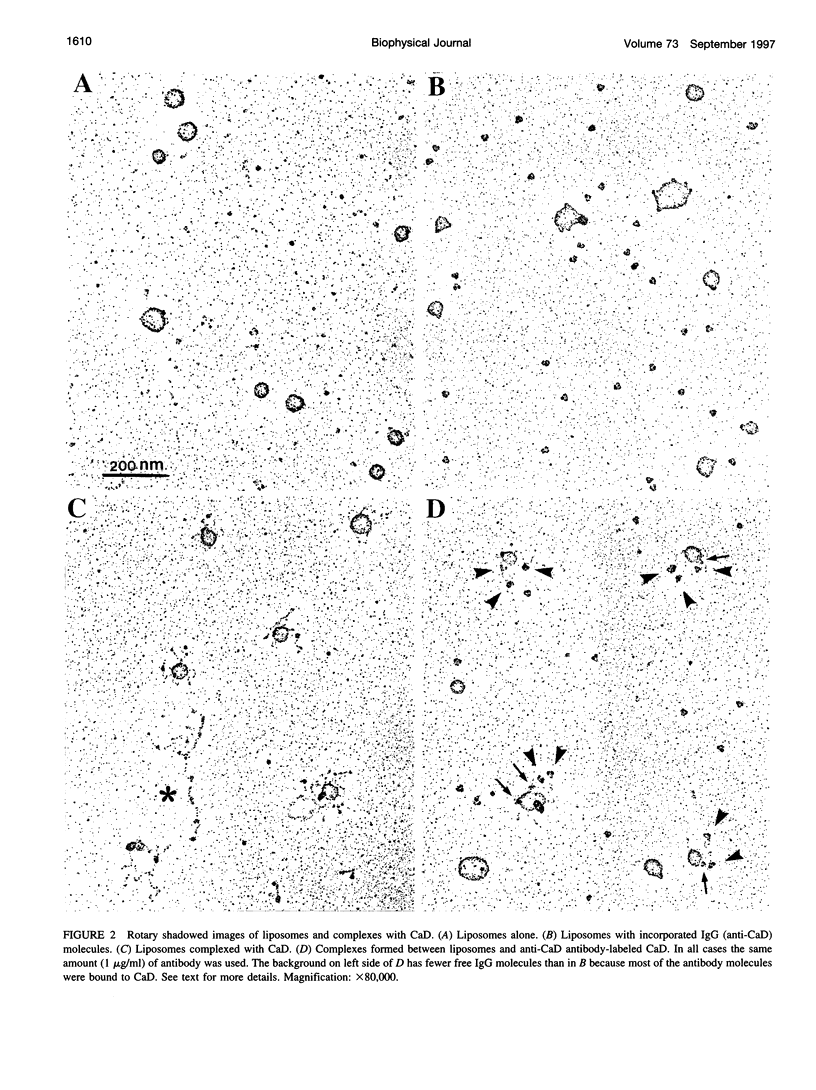
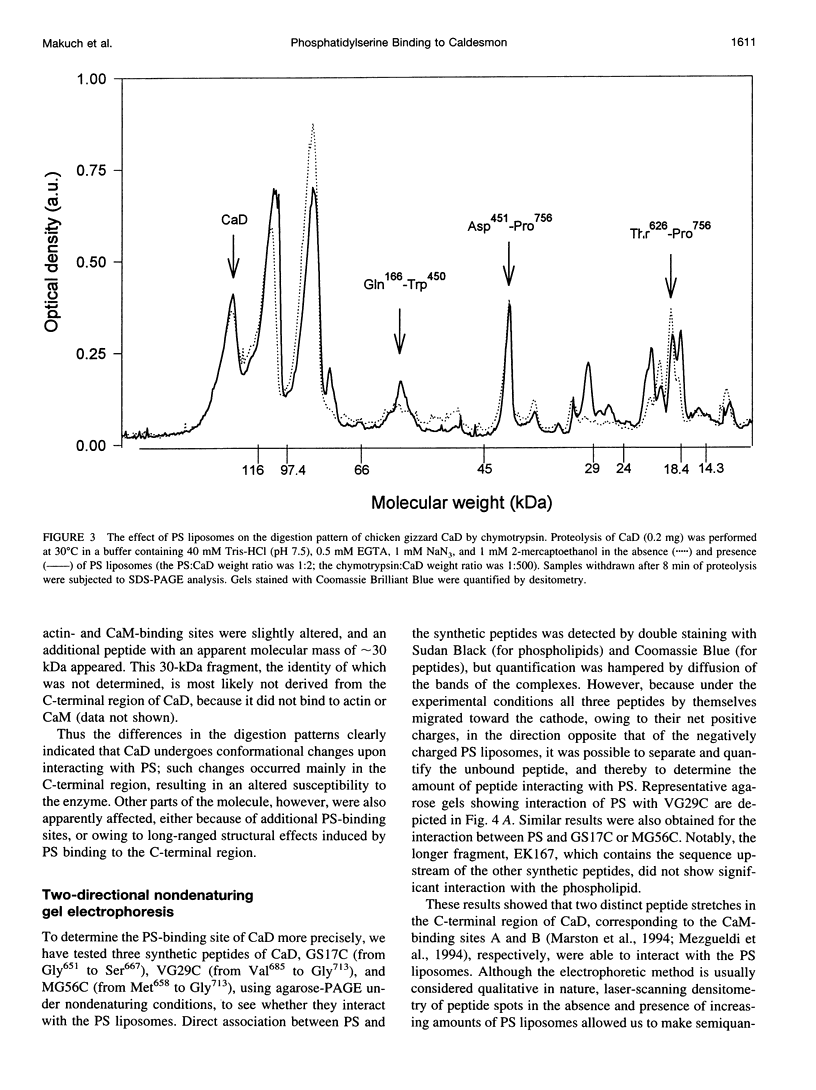
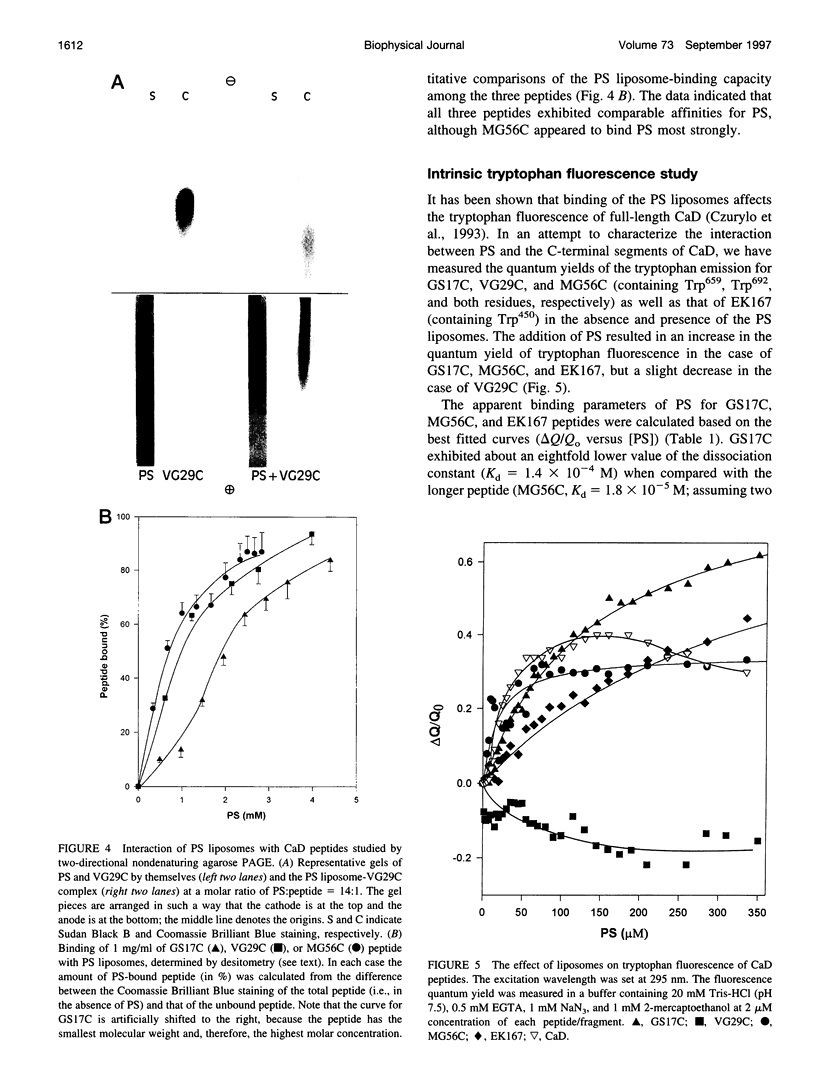
Rotary shadowing electron microscopy revealed that attachment of caldesmon to phosphatidylserine (PS) liposomes was mainly through its C-terminal end. To determine the PS-binding sites of caldesmon, we have made use of synthetic peptides covering the two C-terminal calmodulin binding sites and a recombinant fragment corresponding to the N-terminal end of the C-terminal domain that contains an amphipathic helix. Interactions of these peptides with the PS liposomes were studied by nondenaturing gel electrophoresis and fluorescence spectroscopy. The results showed that both calmodulin-binding sites of caldesmon were able to interact with PS. The affinity (Kd) of PS for these sites was in the range of 1.8-14.3 x 10(-5) M, compared to 0.69 x 10(-5) M for the whole caldesmon molecule. Fragments located outside of calmodulin-binding sites bound PS weakly (3.85 x 10(-4) M) and thus may contain a second class of lipid-binding sites. Binding of PS induced conformational changes in regions other than the C-terminal PS-binding sites, as evidenced by the changes in the susceptibility to proteolytic cleavages. Most significantly, the presence of caldesmon greatly increased binding of PS to F-actin, suggesting that caldesmon may tether PS liposomes to actin filaments. These results raise the possibility that caldesmon-lipid interactions could play a functionally important role in the assembly of contractile filaments near the membranes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderem A. The MARCKS brothers: a family of protein kinase C substrates. Cell. 1992 Nov 27;71(5):713–716. doi: 10.1016/0092-8674(92)90546-o. [DOI] [PubMed] [Google Scholar]
- Baudier J., Deloulme J. C., Van Dorsselaer A., Black D., Matthes H. W. Purification and characterization of a brain-specific protein kinase C substrate, neurogranin (p17). Identification of a consensus amino acid sequence between neurogranin and neuromodulin (GAP43) that corresponds to the protein kinase C phosphorylation site and the calmodulin-binding domain. J Biol Chem. 1991 Jan 5;266(1):229–237. [PubMed] [Google Scholar]
- Bogatcheva N. V., Gusev N. B. Computer-assistant prediction of phospholipid binding sites of caldesmon and calponin. FEBS Lett. 1995 Apr 24;363(3):269–272. doi: 10.1016/0014-5793(95)00328-7. [DOI] [PubMed] [Google Scholar]
- Bogatcheva N. V., Huber P. A., Fraser I. D., Marston S. B., Gusev N. B. Localization of phospholipid-binding sites of caldesmon. FEBS Lett. 1994 Apr 4;342(2):176–180. doi: 10.1016/0014-5793(94)80495-8. [DOI] [PubMed] [Google Scholar]
- Bretscher A., Lynch W. Identification and localization of immunoreactive forms of caldesmon in smooth and nonmuscle cells: a comparison with the distributions of tropomyosin and alpha-actinin. J Cell Biol. 1985 May;100(5):1656–1663. doi: 10.1083/jcb.100.5.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A. Smooth muscle caldesmon. Rapid purification and F-actin cross-linking properties. J Biol Chem. 1984 Oct 25;259(20):12873–12880. [PubMed] [Google Scholar]
- Bryan J., Imai M., Lee R., Moore P., Cook R. G., Lin W. G. Cloning and expression of a smooth muscle caldesmon. J Biol Chem. 1989 Aug 15;264(23):13873–13879. [PubMed] [Google Scholar]
- Burgoyne R. D., Cheek T. R., Norman K. M. Identification of a secretory granule-binding protein as caldesmon. Nature. 1986 Jan 2;319(6048):68–70. doi: 10.1038/319068a0. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Biogenesis: plasma membrane calcium ATPase: 15 years of work on the purified enzyme. FASEB J. 1994 Oct;8(13):993–1002. [PubMed] [Google Scholar]
- Cohen A. M., Liu S. C., Derick L. H., Palek J. Ultrastructural studies of the interaction of spectrin with phosphatidylserine liposomes. Blood. 1986 Oct;68(4):920–926. [PubMed] [Google Scholar]
- Cooper J. A. The role of actin polymerization in cell motility. Annu Rev Physiol. 1991;53:585–605. doi: 10.1146/annurev.ph.53.030191.003101. [DOI] [PubMed] [Google Scholar]
- Czuryło E. A., Zborowski J., Dabrowska R. Interaction of caldesmon with phospholipids. Biochem J. 1993 Apr 15;291(Pt 2):403–408. doi: 10.1042/bj2910403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska R., Goch A., Gałazkiewicz B., Osińska H. The influence of caldesmon on ATPase activity of the skeletal muscle actomyosin and bundling of actin filaments. Biochim Biophys Acta. 1985 Sep 27;842(1):70–75. doi: 10.1016/0304-4165(85)90295-8. [DOI] [PubMed] [Google Scholar]
- Dabrowska R., Hinssen H., Gałazkiewicz B., Nowak E. Modulation of gelsolin-induced actin-filament severing by caldesmon and tropomyosin and the effect of these proteins on the actin activation of myosin Mg(2+)-ATPase activity. Biochem J. 1996 May 1;315(Pt 3):753–759. doi: 10.1042/bj3150753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D., Schwarz E., Komaromy M., Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol. 1984 Oct 15;179(1):125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- Gałazkiewicz B., Belagyi J., Dabrowska R. The effect of caldesmon on assembly and dynamic properties of actin. Eur J Biochem. 1989 May 15;181(3):607–614. doi: 10.1111/j.1432-1033.1989.tb14767.x. [DOI] [PubMed] [Google Scholar]
- Gałazkiewicz B., Buss F., Jockusch B. M., Dabrowska R. Caldesmon-induced polymerization of actin from profilactin. Eur J Biochem. 1991 Jan 30;195(2):543–547. doi: 10.1111/j.1432-1033.1991.tb15735.x. [DOI] [PubMed] [Google Scholar]
- Gałazkiewicz B., Mossakowska M., Osińska H., Dabrowska R. Polymerization of G-actin by caldesmon. FEBS Lett. 1985 May 6;184(1):144–149. doi: 10.1016/0014-5793(85)80671-2. [DOI] [PubMed] [Google Scholar]
- Gicquaud C. Actin conformation is drastically altered by direct interaction with membrane lipids: a differential scanning calorimetry study. Biochemistry. 1993 Nov 9;32(44):11873–11877. doi: 10.1021/bi00095a016. [DOI] [PubMed] [Google Scholar]
- Graceffa P., Wang C. L., Stafford W. F. Caldesmon. Molecular weight and subunit composition by analytical ultracentrifugation. J Biol Chem. 1988 Oct 5;263(28):14196–14202. [PubMed] [Google Scholar]
- Hartwig J. H., Thelen M., Rosen A., Janmey P. A., Nairn A. C., Aderem A. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature. 1992 Apr 16;356(6370):618–622. doi: 10.1038/356618a0. [DOI] [PubMed] [Google Scholar]
- Houbre D., Duportail G., Deloulme J. C., Baudier J. The interactions of the brain-specific calmodulin-binding protein kinase C substrate, neuromodulin (GAP 43), with membrane phospholipids. J Biol Chem. 1991 Apr 15;266(11):7121–7131. [PubMed] [Google Scholar]
- Houk T. W., Jr, Ue K. The measurement of actin concentration in solution: a comparison of methods. Anal Biochem. 1974 Nov;62(1):66–74. doi: 10.1016/0003-2697(74)90367-4. [DOI] [PubMed] [Google Scholar]
- Isenberg G. Actin binding proteins--lipid interactions. J Muscle Res Cell Motil. 1991 Apr;12(2):136–144. doi: 10.1007/BF01774032. [DOI] [PubMed] [Google Scholar]
- Ishikawa R., Yamashiro S., Matsumura F. Annealing of gelsolin-severed actin fragments by tropomyosin in the presence of Ca2+. Potentiation of the annealing process by caldesmon. J Biol Chem. 1989 Oct 5;264(28):16764–16770. [PubMed] [Google Scholar]
- Ishikawa R., Yamashiro S., Matsumura F. Differential modulation of actin-severing activity of gelsolin by multiple isoforms of cultured rat cell tropomyosin. Potentiation of protective ability of tropomyosins by 83-kDa nonmuscle caldesmon. J Biol Chem. 1989 May 5;264(13):7490–7497. [PubMed] [Google Scholar]
- Janmey P. A. Phosphoinositides and calcium as regulators of cellular actin assembly and disassembly. Annu Rev Physiol. 1994;56:169–191. doi: 10.1146/annurev.ph.56.030194.001125. [DOI] [PubMed] [Google Scholar]
- Kim J., Blackshear P. J., Johnson J. D., McLaughlin S. Phosphorylation reverses the membrane association of peptides that correspond to the basic domains of MARCKS and neuromodulin. Biophys J. 1994 Jul;67(1):227–237. doi: 10.1016/S0006-3495(94)80473-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin J. J., Davis-Nanthakumar E. J., Jin J. P., Lourim D., Novy R. E., Lin J. L. Epitope mapping of monoclonal antibodies against caldesmon and their effects on the binding of caldesmon to Ca++/calmodulin and to actin or actin-tropomyosin filaments. Cell Motil Cytoskeleton. 1991;20(2):95–108. doi: 10.1002/cm.970200203. [DOI] [PubMed] [Google Scholar]
- Mabuchi K. Heavy-meromyosin-decorated actin filaments: a simple method to preserve actin filaments for rotary shadowing. J Struct Biol. 1991 Aug;107(1):22–28. doi: 10.1016/1047-8477(91)90027-t. [DOI] [PubMed] [Google Scholar]
- Mabuchi K., Lin J. J., Wang C. L. Electron microscopic images suggest both ends of caldesmon interact with actin filaments. J Muscle Res Cell Motil. 1993 Feb;14(1):54–64. doi: 10.1007/BF00132180. [DOI] [PubMed] [Google Scholar]
- Mabuchi K., Wang C. L. Electron microscopic studies of chicken gizzard caldesmon and its complex with calmodulin. J Muscle Res Cell Motil. 1991 Apr;12(2):145–151. doi: 10.1007/BF01774033. [DOI] [PubMed] [Google Scholar]
- Marston S. B., Fraser I. D., Huber P. A., Pritchard K., Gusev N. B., Torok K. Location of two contact sites between human smooth muscle caldesmon and Ca(2+)-calmodulin. J Biol Chem. 1994 Mar 18;269(11):8134–8139. [PubMed] [Google Scholar]
- Marston S. B., Redwood C. S. The molecular anatomy of caldesmon. Biochem J. 1991 Oct 1;279(Pt 1):1–16. doi: 10.1042/bj2790001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura F., Yamashiro S. Caldesmon. Curr Opin Cell Biol. 1993 Feb;5(1):70–76. doi: 10.1016/s0955-0674(05)80010-9. [DOI] [PubMed] [Google Scholar]
- Mezgueldi M., Derancourt J., Calas B., Kassab R., Fattoum A. Precise identification of the regulatory F-actin- and calmodulin-binding sequences in the 10-kDa carboxyl-terminal domain of caldesmon. J Biol Chem. 1994 Apr 29;269(17):12824–12832. [PubMed] [Google Scholar]
- Rouser G., Fkeischer S., Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970 May;5(5):494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- Sobue K., Sellers J. R. Caldesmon, a novel regulatory protein in smooth muscle and nonmuscle actomyosin systems. J Biol Chem. 1991 Jul 5;266(19):12115–12118. [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- St-Onge D., Gicquaud C. Evidence of direct interaction between actin and membrane lipids. Biochem Cell Biol. 1989 Jun;67(6):297–300. doi: 10.1139/o89-045. [DOI] [PubMed] [Google Scholar]
- Surgucheva I., Bryan J. Over-expression of smooth muscle caldesmon in mouse fibroblasts. Cell Motil Cytoskeleton. 1995;32(3):233–243. doi: 10.1002/cm.970320307. [DOI] [PubMed] [Google Scholar]
- Swanljung-Collins H., Collins J. H. Phosphorylation of brush border myosin I by protein kinase C is regulated by Ca(2+)-stimulated binding of myosin I to phosphatidylserine concerted with calmodulin dissociation. J Biol Chem. 1992 Feb 15;267(5):3445–3454. [PubMed] [Google Scholar]
- Szoka F., Jr, Papahadjopoulos D. Comparative properties and methods of preparation of lipid vesicles (liposomes). Annu Rev Biophys Bioeng. 1980;9:467–508. doi: 10.1146/annurev.bb.09.060180.002343. [DOI] [PubMed] [Google Scholar]
- Szpacenko A., Dabrowska R. Functional domain of caldesmon. FEBS Lett. 1986 Jul 7;202(2):182–186. doi: 10.1016/0014-5793(86)80683-4. [DOI] [PubMed] [Google Scholar]
- Vorotnikov A. V., Bogatcheva N. V., Gusev N. B. Caldesmon-phospholipid interaction. Effect of protein kinase C phosphorylation and sequence similarity with other phospholipid-binding proteins. Biochem J. 1992 Jun 15;284(Pt 3):911–916. doi: 10.1042/bj2840911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorotnikov A. V., Gusev N. B., Hua S., Collins J. H., Redwood C. S., Marston S. B. Phosphorylation of aorta caldesmon by endogenous proteolytic fragments of protein kinase C. J Muscle Res Cell Motil. 1994 Feb;15(1):37–48. doi: 10.1007/BF00123831. [DOI] [PubMed] [Google Scholar]
- Vorotnikov A. V., Gusev N. B. Interaction of smooth muscle caldesmon with phospholipids. FEBS Lett. 1990 Dec 17;277(1-2):134–136. doi: 10.1016/0014-5793(90)80827-6. [DOI] [PubMed] [Google Scholar]
- Walker G., Kerrick W. G., Bourguignon L. Y. The role of caldesmon in the regulation of receptor capping in mouse T-lymphoma cell. J Biol Chem. 1989 Jan 5;264(1):496–500. [PubMed] [Google Scholar]
- Walsh M. P. The Ayerst Award Lecture 1990. Calcium-dependent mechanisms of regulation of smooth muscle contraction. Biochem Cell Biol. 1991 Dec;69(12):771–800. doi: 10.1139/o91-119. [DOI] [PubMed] [Google Scholar]
- Wang C. L., Wang L. W., Xu S. A., Lu R. C., Saavedra-Alanis V., Bryan J. Localization of the calmodulin- and the actin-binding sites of caldesmon. J Biol Chem. 1991 May 15;266(14):9166–9172. [PubMed] [Google Scholar]
- Warren K. S., Lin J. L., Wamboldt D. D., Lin J. J. Overexpression of human fibroblast caldesmon fragment containing actin-, Ca++/calmodulin-, and tropomyosin-binding domains stabilizes endogenous tropomyosin and microfilaments. J Cell Biol. 1994 Apr;125(2):359–368. doi: 10.1083/jcb.125.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro S., Yamakita Y., Hosoya H., Matsumura F. Phosphorylation of non-muscle caldesmon by p34cdc2 kinase during mitosis. Nature. 1991 Jan 10;349(6305):169–172. doi: 10.1038/349169a0. [DOI] [PubMed] [Google Scholar]
- Yamashiro S., Yamakita Y., Ishikawa R., Matsumura F. Mitosis-specific phosphorylation causes 83K non-muscle caldesmon to dissociate from microfilaments. Nature. 1990 Apr 12;344(6267):675–678. doi: 10.1038/344675a0. [DOI] [PubMed] [Google Scholar]
- Zhan Q. Q., Wong S. S., Wang C. L. A calmodulin-binding peptide of caldesmon. J Biol Chem. 1991 Nov 15;266(32):21810–21814. [PubMed] [Google Scholar]
- Zhuang S., Wang E., Wang C. L. Identification of the functionally relevant calmodulin binding site in smooth muscle caldesmon. J Biol Chem. 1995 Aug 25;270(34):19964–19968. doi: 10.1074/jbc.270.34.19964. [DOI] [PubMed] [Google Scholar]