Abstract
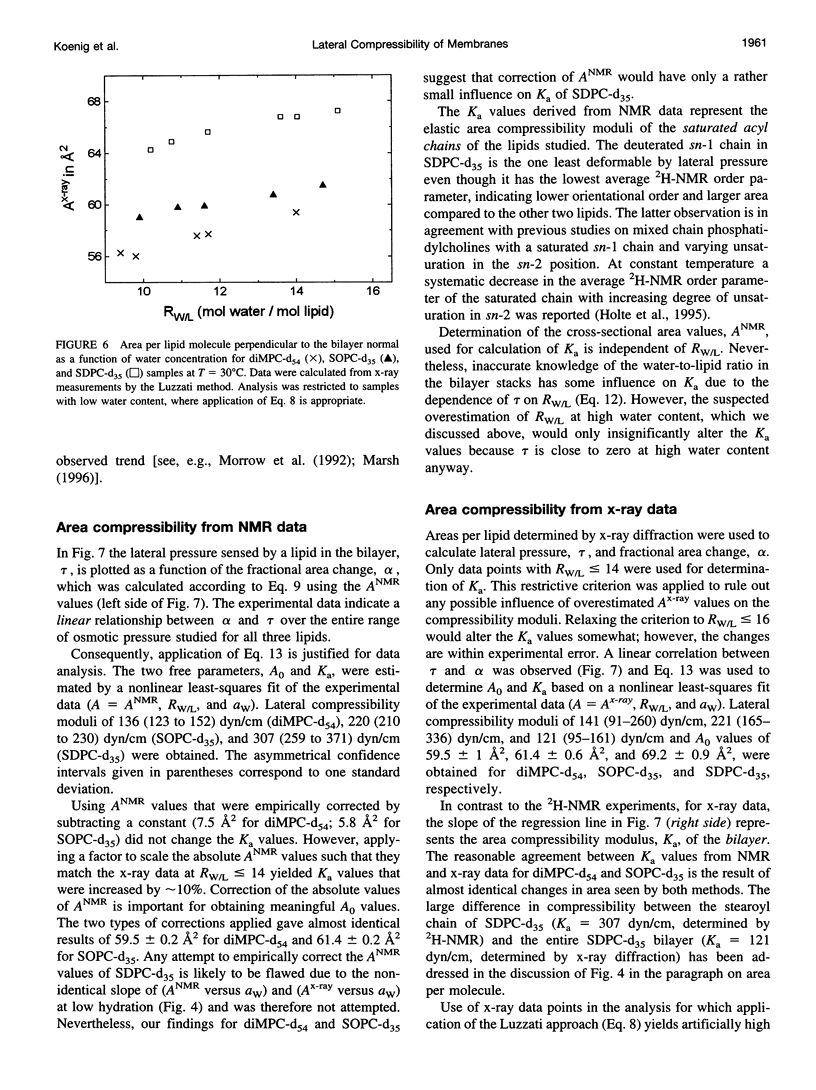
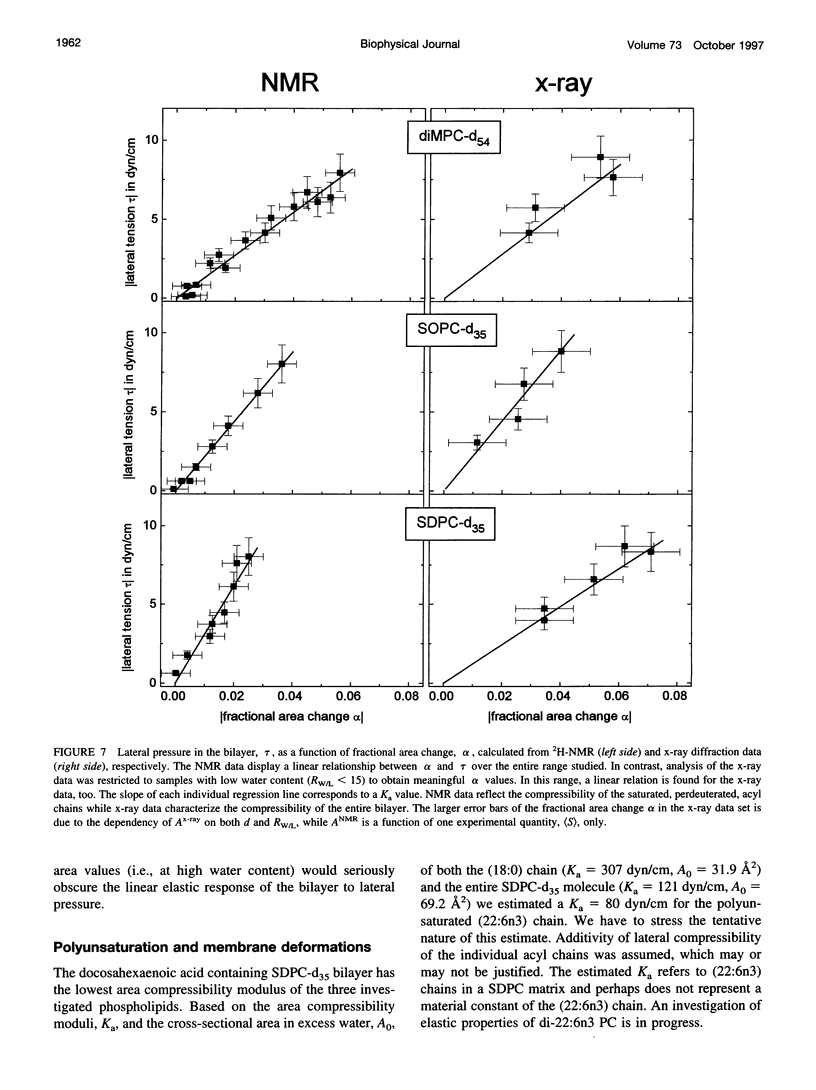
The elastic area compressibility modulus, Ka, of lamellar liquid crystalline bilayers was determined by a new experimental approach using 2H-NMR order parameters of lipid hydrocarbon chains together with lamellar repeat spacings measured by x-ray diffraction. The combination of NMR and x-ray techniques yields accurate determination of lateral area per lipid molecule. Samples of saturated, monounsaturated, and polyunsaturated phospholipids were equilibrated with polyethylene glycol (PEG) 20,000 solutions in water at concentrations from 0 to 55 wt % PEG at 30 degrees C. This procedure is equivalent to applying 0 to 8 dyn/cm lateral pressure to the bilayers. The resulting reductions in area per lipid were measured with a resolution of +/-0.2 A2 and the fractional area decrease was proportional to applied lateral pressure. For 1,2-dimyristoyl(d54)-sn-glycero-3-phosphocholine, 1-stearoyl(d35)-2-oleoyl-sn-glycero-3-phosphocholine (SOPC-d35), and 1-stearoyl(d35)-2-docosahexaenoyl-sn-glycero-3-phosphocholine (SDPC-d35) cross-sectional areas per molecule in excess water of 59.5, 61.4, and 69.2 A2 and bilayer elastic area compressibility moduli of 141, 221, and 121 dyn/cm were determined, respectively. Combining NMR and x-ray results enables the determination of compressibility differences between saturated and unsaturated hydrocarbon chains. In mixed-chain SOPC-d35 both chains have similar compressibility moduli; however, in mixed-chain polyunsaturated SDPC-d35, the saturated stearic acid chain appears to be far less compressible than the polyunsaturated docosahexaenoic acid chain.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attwood P. V., Gutfreund H. The application of pressure relaxation to the study of the equilibrium between metarhodopsin I and II from bovine retinas. FEBS Lett. 1980 Oct 6;119(2):323–326. doi: 10.1016/0014-5793(80)80281-x. [DOI] [PubMed] [Google Scholar]
- Davis J. H. The description of membrane lipid conformation, order and dynamics by 2H-NMR. Biochim Biophys Acta. 1983 Mar 21;737(1):117–171. doi: 10.1016/0304-4157(83)90015-1. [DOI] [PubMed] [Google Scholar]
- Demiel R. A., Guerts van Kessel W. S., van Deenen L. L. The properties of polyunsaturated lecithins in monolayers and liposomes and the interactions of these lecithins with cholesterol. Biochim Biophys Acta. 1972 Apr 14;266(1):26–40. doi: 10.1016/0005-2736(72)90116-2. [DOI] [PubMed] [Google Scholar]
- Evans E. A., Parsegian V. A. Thermal-mechanical fluctuations enhance repulsion between bimolecular layers. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7132–7136. doi: 10.1073/pnas.83.19.7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Kwok R. Mechanical calorimetry of large dimyristoylphosphatidylcholine vesicles in the phase transition region. Biochemistry. 1982 Sep 28;21(20):4874–4879. doi: 10.1021/bi00263a007. [DOI] [PubMed] [Google Scholar]
- Evans E, Rawicz W. Entropy-driven tension and bending elasticity in condensed-fluid membranes. Phys Rev Lett. 1990 Apr 23;64(17):2094–2097. doi: 10.1103/PhysRevLett.64.2094. [DOI] [PubMed] [Google Scholar]
- Ghosh D., Williams M. A., Tinoco J. The influence of lecithin structure on their monolayer behavior and interactions with cholesterol. Biochim Biophys Acta. 1973 Jan 26;291(2):351–362. doi: 10.1016/0005-2736(73)90488-4. [DOI] [PubMed] [Google Scholar]
- Holte L. L., Peter S. A., Sinnwell T. M., Gawrisch K. 2H nuclear magnetic resonance order parameter profiles suggest a change of molecular shape for phosphatidylcholines containing a polyunsaturated acyl chain. Biophys J. 1995 Jun;68(6):2396–2403. doi: 10.1016/S0006-3495(95)80422-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holte L. L., Separovic F., Gawrisch K. Nuclear magnetic resonance investigation of hydrocarbon chain packing in bilayers of polyunsaturated phospholipids. Lipids. 1996 Mar;31 (Suppl):S199–S203. doi: 10.1007/BF02637076. [DOI] [PubMed] [Google Scholar]
- Ipsen J. H., Mouritsen O. G., Bloom M. Relationships between lipid membrane area, hydrophobic thickness, and acyl-chain orientational order. The effects of cholesterol. Biophys J. 1990 Mar;57(3):405–412. doi: 10.1016/S0006-3495(90)82557-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jendrasiak G. L., Hasty J. H. The hydration of phospholipids. Biochim Biophys Acta. 1974 Jan 23;337(1):79–91. doi: 10.1016/0005-2760(74)90042-3. [DOI] [PubMed] [Google Scholar]
- Johnson M. L. The analysis of ligand-binding data with experimental uncertainties in the independent variables. Anal Biochem. 1985 Aug 1;148(2):471–478. doi: 10.1016/0003-2697(85)90254-4. [DOI] [PubMed] [Google Scholar]
- Kwok R., Evans E. Thermoelasticity of large lecithin bilayer vesicles. Biophys J. 1981 Sep;35(3):637–652. doi: 10.1016/S0006-3495(81)84817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafleur M., Fine B., Sternin E., Cullis P. R., Bloom M. Smoothed orientational order profile of lipid bilayers by 2H-nuclear magnetic resonance. Biophys J. 1989 Nov;56(5):1037–1041. doi: 10.1016/S0006-3495(89)82749-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamola A. A., Yamane T., Zipp A. Effects of detergents and high pressures upon the metarhodopsin I--metarhodopsin II equilibrium. Biochemistry. 1974 Feb 12;13(4):738–745. doi: 10.1021/bi00701a016. [DOI] [PubMed] [Google Scholar]
- Leikin S., Parsegian V. A., Rau D. C., Rand R. P. Hydration forces. Annu Rev Phys Chem. 1993;44:369–395. doi: 10.1146/annurev.pc.44.100193.002101. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Parker K. R., Dratz E. A. The molecular mechanism of visual excitation and its relation to the structure and composition of the rod outer segment. Annu Rev Physiol. 1987;49:765–791. doi: 10.1146/annurev.ph.49.030187.004001. [DOI] [PubMed] [Google Scholar]
- Lis L. J., McAlister M., Fuller N., Rand R. P., Parsegian V. A. Measurement of the lateral compressibility of several phospholipid bilayers. Biophys J. 1982 Mar;37(3):667–672. [PMC free article] [PubMed] [Google Scholar]
- Marsh D. Lateral pressure in membranes. Biochim Biophys Acta. 1996 Oct 29;1286(3):183–223. doi: 10.1016/s0304-4157(96)00009-3. [DOI] [PubMed] [Google Scholar]
- McGee C. D., Greenwood C. E. Effects of dietary fatty acid composition on macronutrient selection and synaptosomal fatty acid composition in rats. J Nutr. 1989 Nov;119(11):1561–1568. doi: 10.1093/jn/119.11.1561. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Advani S., Burton R. E., Zhelev D. V., Needham D., Simon S. A. Experimental tests for protrusion and undulation pressures in phospholipid bilayers. Biochemistry. 1995 Jul 11;34(27):8520–8532. doi: 10.1021/bi00027a002. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A. Hydration force and bilayer deformation: a reevaluation. Biochemistry. 1986 Jul 15;25(14):4058–4066. doi: 10.1021/bi00362a011. [DOI] [PubMed] [Google Scholar]
- Miljanich G. P., Sklar L. A., White D. L., Dratz E. A. Disaturated and dipolyunsaturated phospholipids in the bovine retinal rod outer segment disk membrane. Biochim Biophys Acta. 1979 Apr 4;552(2):294–306. doi: 10.1016/0005-2736(79)90284-0. [DOI] [PubMed] [Google Scholar]
- Mitchell D. C., Straume M., Litman B. J. Role of sn-1-saturated,sn-2-polyunsaturated phospholipids in control of membrane receptor conformational equilibrium: effects of cholesterol and acyl chain unsaturation on the metarhodopsin I in equilibrium with metarhodopsin II equilibrium. Biochemistry. 1992 Jan 28;31(3):662–670. doi: 10.1021/bi00118a005. [DOI] [PubMed] [Google Scholar]
- Morrow M. R., Whitehead J. P., Lu D. Chain-length dependence of lipid bilayer properties near the liquid crystal to gel phase transition. Biophys J. 1992 Jul;63(1):18–27. doi: 10.1016/S0006-3495(92)81579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle J. F. Area/lipid of bilayers from NMR. Biophys J. 1993 May;64(5):1476–1481. doi: 10.1016/S0006-3495(93)81514-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle J. F., Wilkinson D. A. Lecithin bilayers. Density measurement and molecular interactions. Biophys J. 1978 Aug;23(2):159–175. doi: 10.1016/S0006-3495(78)85441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle J. F., Zhang R., Tristram-Nagle S., Sun W., Petrache H. I., Suter R. M. X-ray structure determination of fully hydrated L alpha phase dipalmitoylphosphatidylcholine bilayers. Biophys J. 1996 Mar;70(3):1419–1431. doi: 10.1016/S0006-3495(96)79701-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham D., Evans E. Structure and mechanical properties of giant lipid (DMPC) vesicle bilayers from 20 degrees C below to 10 degrees C above the liquid crystal-crystalline phase transition at 24 degrees C. Biochemistry. 1988 Oct 18;27(21):8261–8269. doi: 10.1021/bi00421a041. [DOI] [PubMed] [Google Scholar]
- Needham D., Nunn R. S. Elastic deformation and failure of lipid bilayer membranes containing cholesterol. Biophys J. 1990 Oct;58(4):997–1009. doi: 10.1016/S0006-3495(90)82444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien D. F., Costa L. F., Ott R. A. Photochemical functionality of rhodopsin-phospholipid recombinant membranes. Biochemistry. 1977 Apr 5;16(7):1295–1303. doi: 10.1021/bi00626a009. [DOI] [PubMed] [Google Scholar]
- Parsegian V. A., Fuller N., Rand R. P. Measured work of deformation and repulsion of lecithin bilayers. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2750–2754. doi: 10.1073/pnas.76.6.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsegian V. A., Rand R. P., Fuller N. L., Rau D. C. Osmotic stress for the direct measurement of intermolecular forces. Methods Enzymol. 1986;127:400–416. doi: 10.1016/0076-6879(86)27032-9. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Fuller N., Parsegian V. A., Rau D. C. Variation in hydration forces between neutral phospholipid bilayers: evidence for hydration attraction. Biochemistry. 1988 Oct 4;27(20):7711–7722. doi: 10.1021/bi00420a021. [DOI] [PubMed] [Google Scholar]
- Schindler H., Seelig J. Deuterium order parameters in relation to thermodynamic properties of a phospholiped bilayer. A statistical mechanical interpretation. Biochemistry. 1975 Jun 3;14(11):2283–2287. doi: 10.1021/bi00682a001. [DOI] [PubMed] [Google Scholar]
- Schneider M. B., Jenkins J. T., Webb W. W. Thermal fluctuations of large cylindrical phospholipid vesicles. Biophys J. 1984 May;45(5):891–899. doi: 10.1016/S0006-3495(84)84235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig A., Seelig J. The dynamic structure of fatty acyl chains in a phospholipid bilayer measured by deuterium magnetic resonance. Biochemistry. 1974 Nov 5;13(23):4839–4845. doi: 10.1021/bi00720a024. [DOI] [PubMed] [Google Scholar]
- Seelig J. Deuterium magnetic resonance: theory and application to lipid membranes. Q Rev Biophys. 1977 Aug;10(3):353–418. doi: 10.1017/s0033583500002948. [DOI] [PubMed] [Google Scholar]
- Seelig J., Seelig A. Lipid conformation in model membranes and biological membranes. Q Rev Biophys. 1980 Feb;13(1):19–61. doi: 10.1017/s0033583500000305. [DOI] [PubMed] [Google Scholar]
- Servuss R. M., Harbich W., Helfrich W. Measurement of the curvature-elastic modulus of egg lecithin bilayers. Biochim Biophys Acta. 1976 Jul 15;436(4):900–903. doi: 10.1016/0005-2736(76)90422-3. [DOI] [PubMed] [Google Scholar]
- Straume M., Litman B. J. Equilibrium and dynamic structure of large, unilamellar, unsaturated acyl chain phosphatidylcholine vesicles. Higher order analysis of 1,6-diphenyl-1,3,5-hexatriene and 1-[4-(trimethylammonio)phenyl]- 6-phenyl-1,3,5-hexatriene anisotropy decay. Biochemistry. 1987 Aug 11;26(16):5113–5120. doi: 10.1021/bi00390a033. [DOI] [PubMed] [Google Scholar]
- Straume M., Litman B. J. Influence of cholesterol on equilibrium and dynamic bilayer structure of unsaturated acyl chain phosphatidylcholine vesicles as determined from higher order analysis of fluorescence anisotropy decay. Biochemistry. 1987 Aug 11;26(16):5121–5126. doi: 10.1021/bi00390a034. [DOI] [PubMed] [Google Scholar]
- White S. H., Jacobs R. E., King G. I. Partial specific volumes of lipid and water in mixtures of egg lecithin and water. Biophys J. 1987 Oct;52(4):663–665. doi: 10.1016/S0006-3495(87)83259-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedmann T. S., Pates R. D., Beach J. M., Salmon A., Brown M. F. Lipid-protein interactions mediate the photochemical function of rhodopsin. Biochemistry. 1988 Aug 23;27(17):6469–6474. doi: 10.1021/bi00417a041. [DOI] [PubMed] [Google Scholar]
- Wiener M. C., White S. H. Fluid bilayer structure determination by the combined use of x-ray and neutron diffraction. I. Fluid bilayer models and the limits of resolution. Biophys J. 1991 Jan;59(1):162–173. doi: 10.1016/S0006-3495(91)82208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]




