Abstract
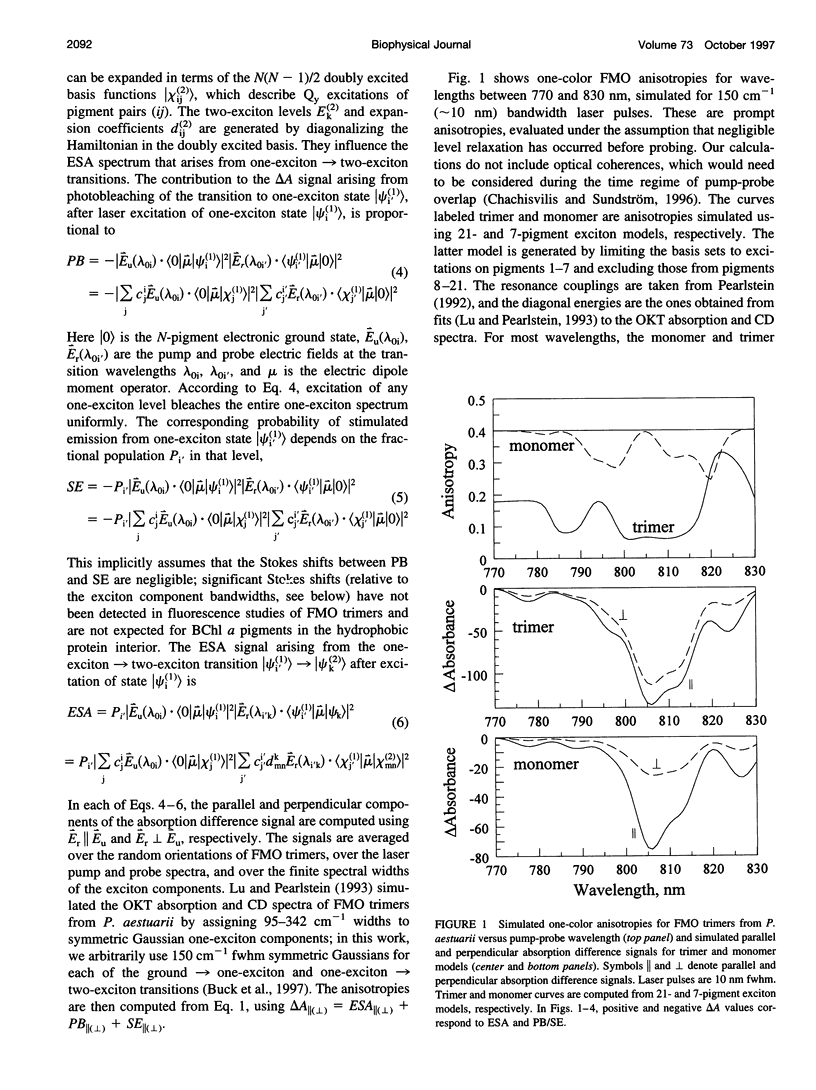
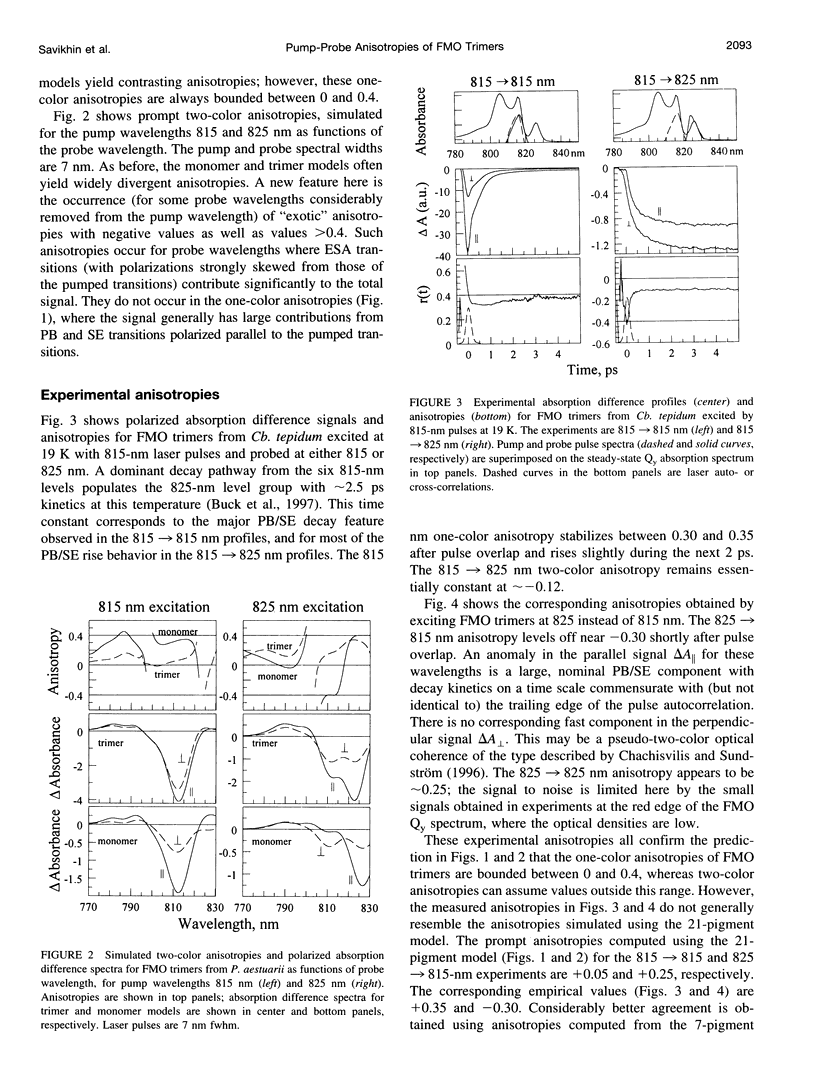
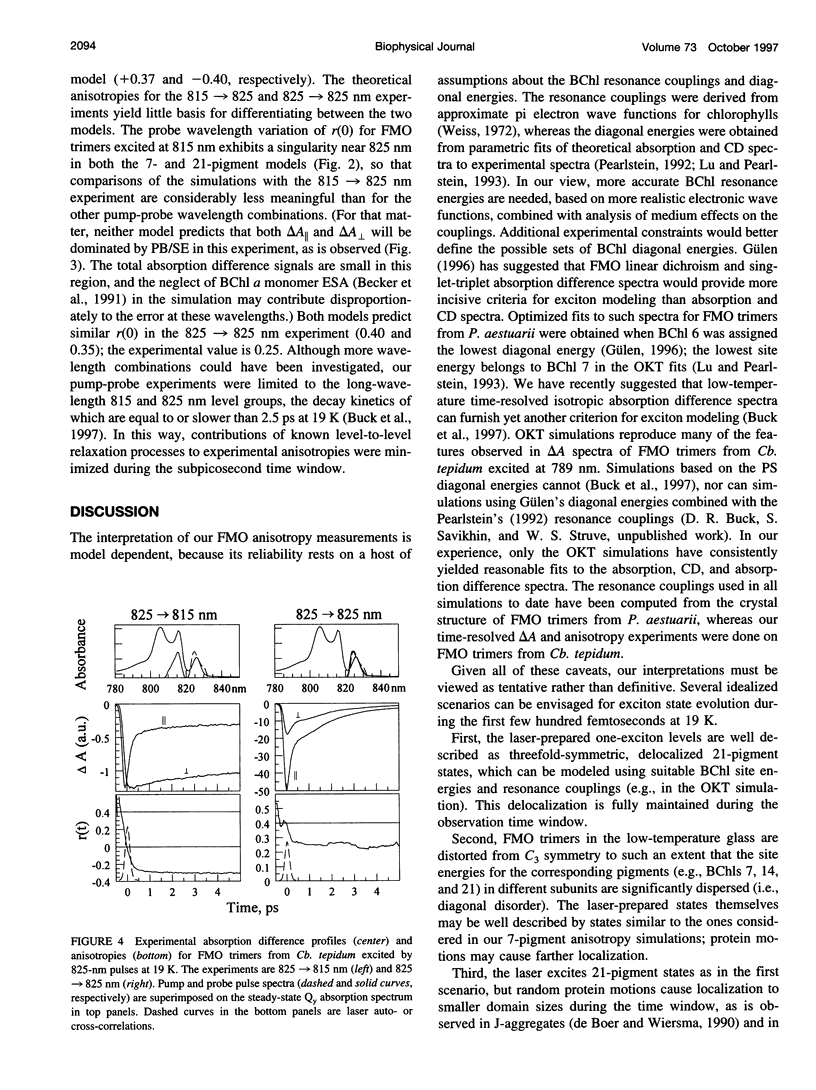
Exciton calculations on symmetric and asymmetric Fenna-Matthews-Olson (FMO) trimers, combined with absorption difference anisotropy measurements on FMO trimers from the green bacterium Chlorobium tepidum, suggest that real samples exhibit sufficient diagonal energy disorder so that their laser-excited exciton states are noticeably localized. Our observed anisotropies are clearly inconsistent with 21-pigment exciton simulations based on a threefold-symmetric FMO protein. They are more consistent with a 7-pigment model that assumes that the laser-prepared states are localized within a subunit of the trimer. Differential diagonal energy shifts of 50 cm(-1) between symmetry-related pigments in different subunits are large enough to cause sharp localization in the stationary states; these shifts are commensurate with the approximately 95 cm(-1) inhomogeneous linewidth of the lowest exciton levels. Experimental anisotropies (and by implication steady-state linear and circular dichroism) likely arise from statistical averaging over states with widely contrasting values of these observables, in consequence of their sensitivity to diagonal energy disorder.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blankenship R. E., Cheng P., Causgrove T. P., Brune D. C., Wang SH-H, Choh J-U, Wang J. Redox regulation of energy transfer efficiency in antennas of green photosynthetic bacteria. Photochem Photobiol. 1993;57(1):103–107. doi: 10.1111/j.1751-1097.1993.tb02263.x. [DOI] [PubMed] [Google Scholar]
- Buck D. R., Savikhin S., Struve W. S. Ultrafast absorption difference spectra of the Fenna-Matthews-Olson protein at 19 K: experiment and simulations. Biophys J. 1997 Jan;72(1):24–36. doi: 10.1016/S0006-3495(97)78644-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daurat-Larroque S. T., Brew K., Fenna R. E. The complete amino acid sequence of a bacteriochlorophyll a-protein from Prosthecochloris aestuarii. J Biol Chem. 1986 Mar 15;261(8):3607–3615. [PubMed] [Google Scholar]
- Olson J. M. Chlorophyll organization in green photosynthetic bacteria. Biochim Biophys Acta. 1980 Dec 22;594(1):33–51. doi: 10.1016/0304-4173(80)90012-9. [DOI] [PubMed] [Google Scholar]
- Olson J. M., Ke B., Thompson K. H. Exciton interaction among chlorophyll molecules in bacteriochlorophyllaproteins and bacteriochlorophyllareaction center complexes from green bacteria. Biochim Biophys Acta. 1976 Jun 8;430(3):524–537. doi: 10.1016/0005-2728(76)90028-1. [DOI] [PubMed] [Google Scholar]
- Philipson K. D., Sauer K. Exciton interaction in a bacteriochlorophyll--protein from Chloropseudomonas ethylica. Absorption and circular dichroism at 77 degrees K. Biochemistry. 1972 May 9;11(10):1880–1885. doi: 10.1021/bi00760a024. [DOI] [PubMed] [Google Scholar]
- Savikhin S., Struve W. S. Ultrafast energy transfer in FMO trimers from the green bacterium Chlorobium tepidum. Biochemistry. 1994 Sep 20;33(37):11200–11208. doi: 10.1021/bi00203a016. [DOI] [PubMed] [Google Scholar]
- Savikhin S., Zhou W., Blankenship R. E., Struve W. S. Femtosecond energy transfer and spectral equilibration in bacteriochlorophyll a--protein antenna trimers from the green bacterium Chlorobium tepidum. Biophys J. 1994 Jan;66(1):110–113. doi: 10.1016/S0006-3495(94)80769-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronrud D. E., Schmid M. F., Matthews B. W. Structure and X-ray amino acid sequence of a bacteriochlorophyll A protein from Prosthecochloris aestuarii refined at 1.9 A resolution. J Mol Biol. 1986 Apr 5;188(3):443–454. doi: 10.1016/0022-2836(86)90167-1. [DOI] [PubMed] [Google Scholar]


