Abstract
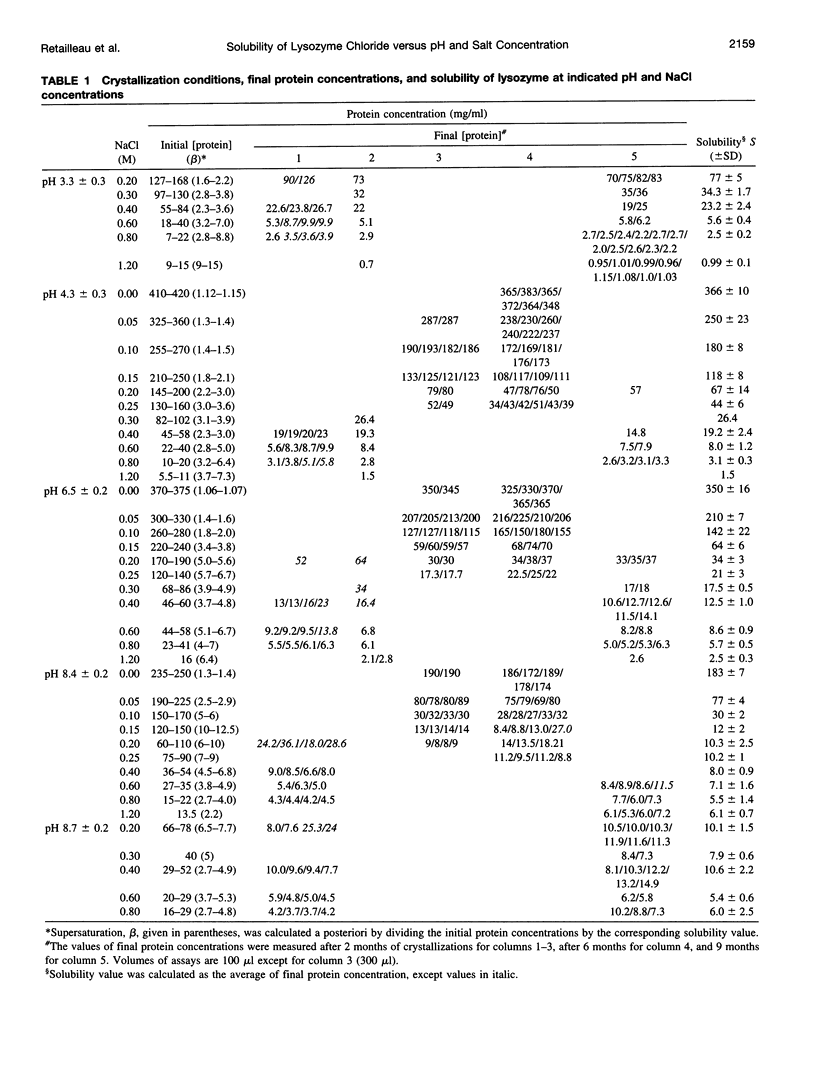
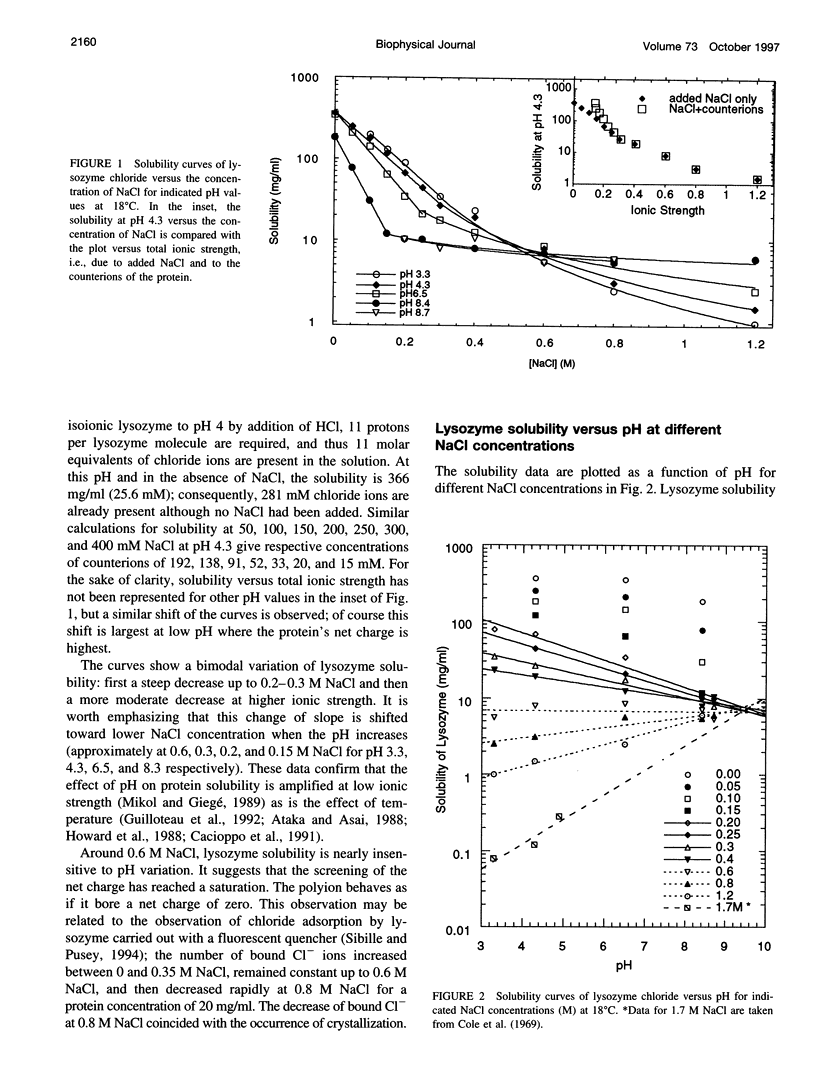
Solubility of lysozyme chloride was determined in the absence of added salt and in the presence of 0.05-1.2 M NaCl, starting from isoionic lysozyme, which was then brought to pH values from 9 to 3 by addition of HCl. The main observation is the absence of a salting-in region whatever the pH studied. This is explained by a predominant electrostatic screening of the positively charged protein and/or by adsorption of chloride ions by the protein. The solubility increases with the protein net charge at low ionic strength, but the reverse is observed at high ionic strength. The solubility of lysozyme chloride seems to become independent of ionic strength at pH approximately 9.5, which is interpreted as a shift of the isoionic pH (10.8) to an isoelectric pH due to chloride binding. The crystallization at very low ionic strength, where lysozyme crystallizes at supersaturation values as low as 1.1, amplifies the effect of pH on protein solubility. Understanding the effect of the net charge and of ionic strength on protein-protein interactions is valuable not only for protein crystal growth but more generally also for the formation of protein-protein or protein-ligand complexes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakawa T., Timasheff S. N. Theory of protein solubility. Methods Enzymol. 1985;114:49–77. doi: 10.1016/0076-6879(85)14005-x. [DOI] [PubMed] [Google Scholar]
- Briggs J. M. Protein-protein diffusional encounter. Biophys J. 1997 May;72(5):1915–1916. doi: 10.1016/S0006-3495(97)78837-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J. B., Bryan M. L. Thermodynamics of solution of lysozyme crystals. Arch Biochem Biophys. 1969 Mar;130(1):86–91. doi: 10.1016/0003-9861(69)90013-7. [DOI] [PubMed] [Google Scholar]
- Crosio M. P., Janin J., Jullien M. Crystal packing in six crystal forms of pancreatic ribonuclease. J Mol Biol. 1992 Nov 5;228(1):243–251. doi: 10.1016/0022-2836(92)90503-c. [DOI] [PubMed] [Google Scholar]
- Israelachvili J., Wennerström H. Role of hydration and water structure in biological and colloidal interactions. Nature. 1996 Jan 18;379(6562):219–225. doi: 10.1038/379219a0. [DOI] [PubMed] [Google Scholar]
- Jolivalt C., Riès-Kautt M., Chevallier P., Ducruix A. X-ray fluorescence used to characterize the salt content of proteins. J Synchrotron Radiat. 1997 Jan 1;4(Pt 1):28–35. doi: 10.1107/S0909049596012769. [DOI] [PubMed] [Google Scholar]
- Leavis P. C., Rothstein F. The solubility of fibrinogen in dilute salt solutions. Arch Biochem Biophys. 1974 Apr 2;161(2):671–682. doi: 10.1016/0003-9861(74)90353-1. [DOI] [PubMed] [Google Scholar]
- Melander W., Horváth C. Salt effect on hydrophobic interactions in precipitation and chromatography of proteins: an interpretation of the lyotropic series. Arch Biochem Biophys. 1977 Sep;183(1):200–215. doi: 10.1016/0003-9861(77)90434-9. [DOI] [PubMed] [Google Scholar]
- Ries-Kautt M. M., Ducruix A. F. Relative effectiveness of various ions on the solubility and crystal growth of lysozyme. J Biol Chem. 1989 Jan 15;264(2):745–748. [PubMed] [Google Scholar]
- Riès-Kautt M., Ducruix A., Van Dorsselaer A. Crystallization of previously desalted lysozyme in the presence of sulfate ions. Acta Crystallogr D Biol Crystallogr. 1994 Jul 1;50(Pt 4):366–369. doi: 10.1107/S0907444994001320. [DOI] [PubMed] [Google Scholar]
- Rosenbaum D, Zamora PC, Zukoski CF. Phase behavior of small attractive colloidal particles. Phys Rev Lett. 1996 Jan 1;76(1):150–153. doi: 10.1103/PhysRevLett.76.150. [DOI] [PubMed] [Google Scholar]
- Schreiber G., Fersht A. R. Rapid, electrostatically assisted association of proteins. Nat Struct Biol. 1996 May;3(5):427–431. doi: 10.1038/nsb0596-427. [DOI] [PubMed] [Google Scholar]
- Sibille L., Pusey M. L. Investigation of nucleating lysozyme solutions. Acta Crystallogr D Biol Crystallogr. 1994 Jul 1;50(Pt 4):396–397. doi: 10.1107/S0907444993013447. [DOI] [PubMed] [Google Scholar]
- TREECE J. M., SHEINSON R. S., MCMEEKIN T. L. THE SOLUBILITIES OF BETA-LACTOGLOBULINS A, B, AND AB. Arch Biochem Biophys. 1964 Oct;108:99–108. doi: 10.1016/0003-9861(64)90360-1. [DOI] [PubMed] [Google Scholar]
- Thomas B. R., Vekilov P. G., Rosenberger F. Heterogeneity determination and purification of commercial hen egg-white lysozyme. Acta Crystallogr D Biol Crystallogr. 1996 Jul 1;52(Pt 4):776–784. doi: 10.1107/S090744499600279X. [DOI] [PubMed] [Google Scholar]
- Vaney M. C., Maignan S., Riès-Kautt M., Ducriux A. High-resolution structure (1.33 A) of a HEW lysozyme tetragonal crystal grown in the APCF apparatus. Data and structural comparison with a crystal grown under microgravity from SpaceHab-01 mission. Acta Crystallogr D Biol Crystallogr. 1996 May 1;52(Pt 3):505–517. doi: 10.1107/S090744499501674X. [DOI] [PubMed] [Google Scholar]
- Vekilov P. G., Monaco B. R., Thomas B. R., Stojanoff V., Rosenberger F. Repartitioning of NaCl and protein impurities in lysozyme crystallization. Acta Crystallogr D Biol Crystallogr. 1996 Jul 1;52(Pt 4):785–798. doi: 10.1107/S0907444996003265. [DOI] [PubMed] [Google Scholar]
- Vérétout F., Delaye M., Tardieu A. Molecular basis of eye lens transparency. Osmotic pressure and X-ray analysis of alpha-crystallin solutions. J Mol Biol. 1989 Feb 20;205(4):713–728. doi: 10.1016/0022-2836(89)90316-1. [DOI] [PubMed] [Google Scholar]


