Abstract
Collagen is the primary structural element in extracellular matrices. In the form of fibers it acts to transmit forces, dissipate energy, and prevent premature mechanical failure in normal tissues. Deformation of collagen fibers involves molecular stretching and slippage, fibrillar slippage, and, ultimately, defibrillation. Our laboratory has developed a process for self-assembly of macroscopic collagen fibers that have structures and mechanical properties similar to rat tail tendon fibers. The purpose of this study is to determine the effects of subfibrillar orientation and decorin incorporation on the mechanical properties of collagen fibers. Self-assembled collagen fibers were stretched 0-50% before cross-linking and then characterized by microscopy and mechanical testing. Results of these studies indicate that fibrillar orientation, packing, and ultimate tensile strength can be increased by stretching. In addition, it is shown that decorin incorporation increases ultimate tensile strength of uncross-linked fibers. Based on the observed results it is hypothesized that decorin facilitates fibrillar slippage during deformation and thereby improves the tensile properties of collagen fibers.
Full text
PDF


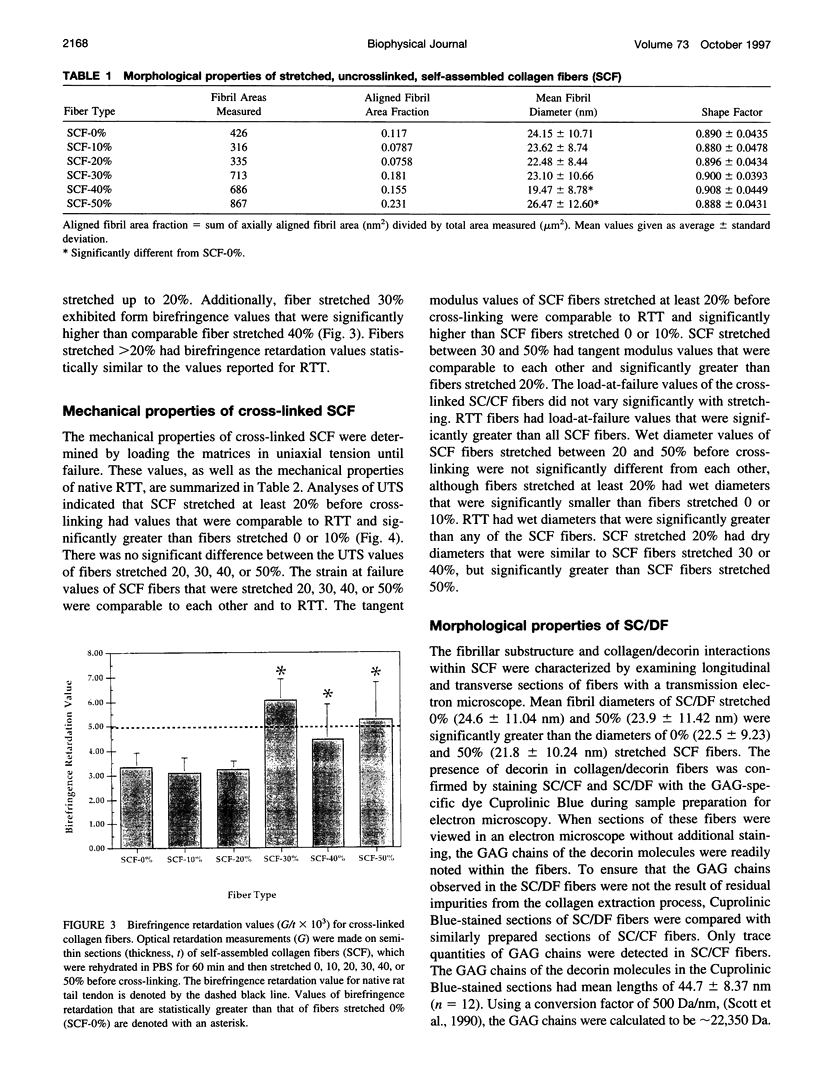
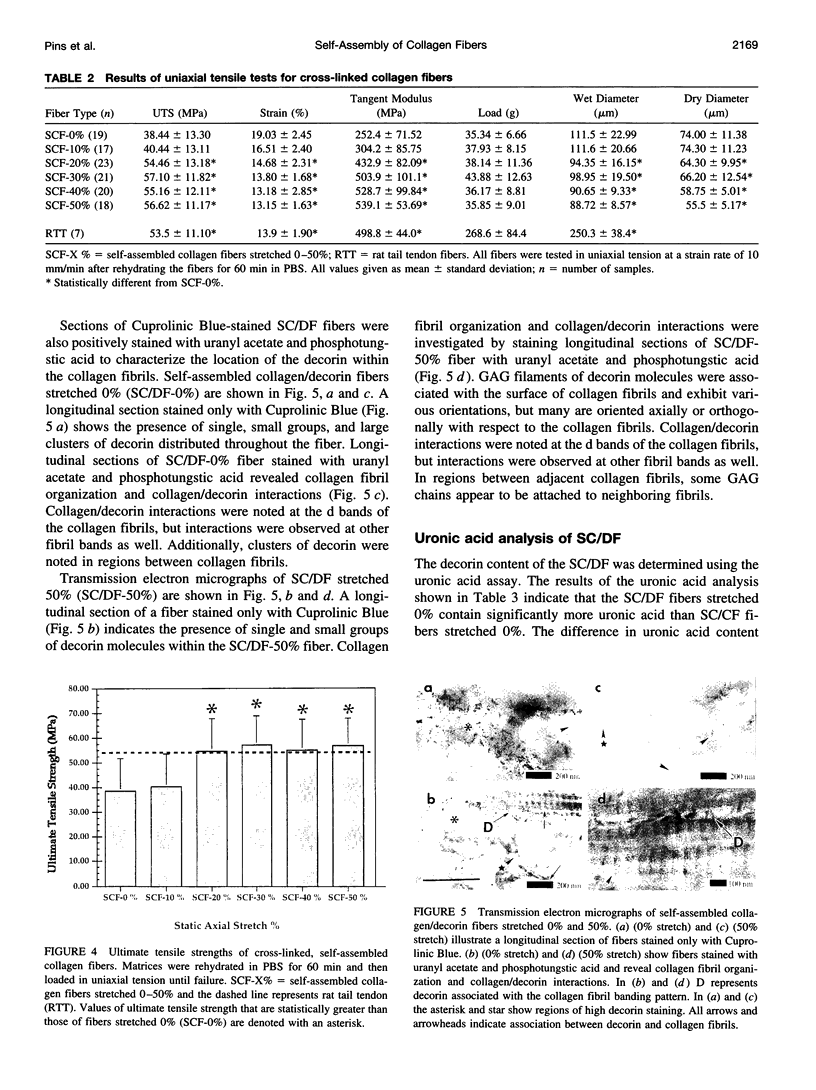
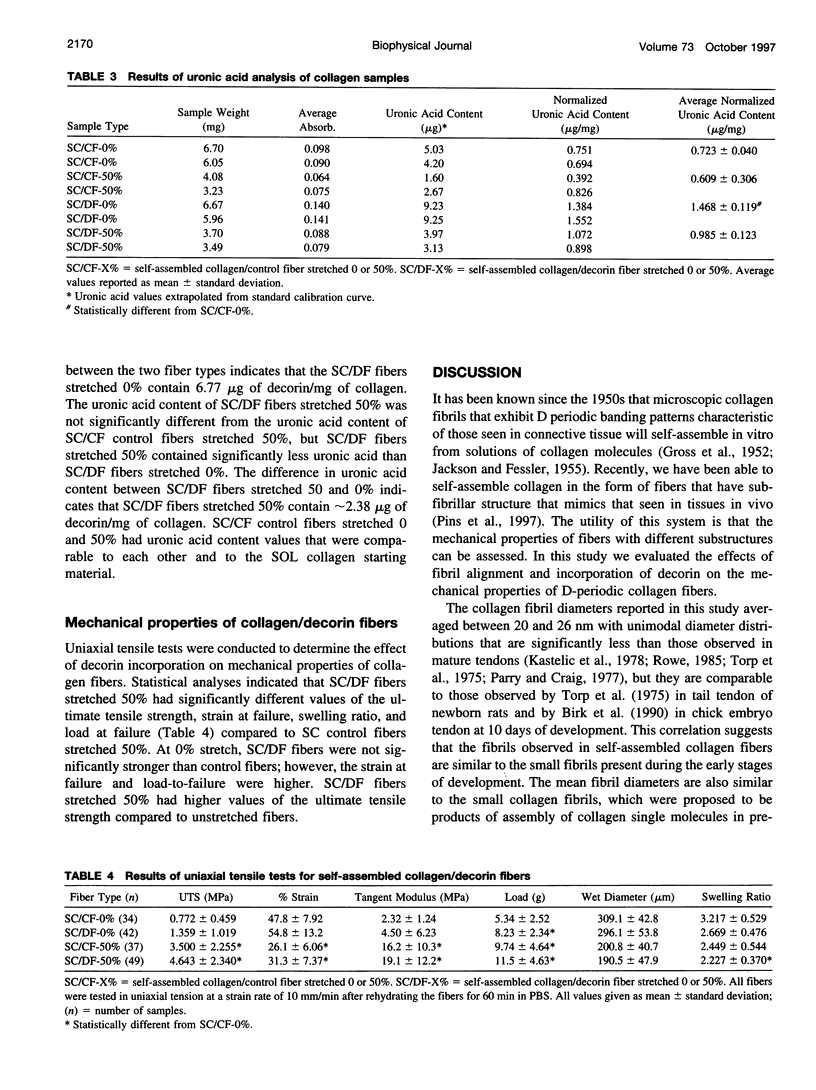


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bell E., Ivarsson B., Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1274–1278. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk D. E., Zycband E. I., Winkelmann D. A., Trelstad R. L. Collagen fibrillogenesis in situ. Discontinuous segmental assembly in extracellular compartments. Ann N Y Acad Sci. 1990;580:176–194. doi: 10.1111/j.1749-6632.1990.tb17928.x. [DOI] [PubMed] [Google Scholar]
- Birk D. E., Zycband E. Assembly of the tendon extracellular matrix during development. J Anat. 1994 Jun;184(Pt 3):457–463. [PMC free article] [PubMed] [Google Scholar]
- Brown D. C., Vogel K. G. Characteristics of the in vitro interaction of a small proteoglycan (PG II) of bovine tendon with type I collagen. Matrix. 1989;9(6):468–478. doi: 10.1016/s0934-8832(11)80016-8. [DOI] [PubMed] [Google Scholar]
- Choi H. U., Johnson T. L., Pal S., Tang L. H., Rosenberg L., Neame P. J. Characterization of the dermatan sulfate proteoglycans, DS-PGI and DS-PGII, from bovine articular cartilage and skin isolated by octyl-sepharose chromatography. J Biol Chem. 1989 Feb 15;264(5):2876–2884. [PubMed] [Google Scholar]
- Cribb A. M., Scott J. E. Tendon response to tensile stress: an ultrastructural investigation of collagen:proteoglycan interactions in stressed tendon. J Anat. 1995 Oct;187(Pt 2):423–428. [PMC free article] [PubMed] [Google Scholar]
- Doillon C. J., Dunn M. G., Bender E., Silver F. H. Collagen fiber formation in repair tissue: development of strength and toughness. Coll Relat Res. 1985 Dec;5(6):481–492. doi: 10.1016/s0174-173x(85)80002-9. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R., Perlish J. S., Faraggiana T. Rotary shadowing of collagen monomers, oligomers, and fibrils during tendon fibrillogenesis. J Histochem Cytochem. 1991 Jan;39(1):51–58. doi: 10.1177/39.1.1983873. [DOI] [PubMed] [Google Scholar]
- GROSS J., HIGHBERGER J. H., SCHMITT F. O. Some factors involved in the fibrogenesis of collagen in vitro. Proc Soc Exp Biol Med. 1952 Jul;80(3):462–465. doi: 10.3181/00379727-80-19657. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- JACKSON D. S., FESSLER J. H. Isolation and properties of a collagen soluble in salt solution at neutral pH. Nature. 1955 Jul 9;176(4471):69–70. doi: 10.1038/176069a0. [DOI] [PubMed] [Google Scholar]
- Kastelic J., Galeski A., Baer E. The multicomposite structure of tendon. Connect Tissue Res. 1978;6(1):11–23. doi: 10.3109/03008207809152283. [DOI] [PubMed] [Google Scholar]
- Kato Y. P., Christiansen D. L., Hahn R. A., Shieh S. J., Goldstein J. D., Silver F. H. Mechanical properties of collagen fibres: a comparison of reconstituted and rat tail tendon fibres. Biomaterials. 1989 Jan;10(1):38–42. doi: 10.1016/0142-9612(89)90007-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mow V. C., Mak A. F., Lai W. M., Rosenberg L. C., Tang L. H. Viscoelastic properties of proteoglycan subunits and aggregates in varying solution concentrations. J Biomech. 1984;17(5):325–338. doi: 10.1016/0021-9290(84)90027-7. [DOI] [PubMed] [Google Scholar]
- Parry D. A., Craig A. S. Quantitative electron microscope observations of the collagen fibrils in rat-tail tendon. Biopolymers. 1977 May;16(5):1015–1031. doi: 10.1002/bip.1977.360160506. [DOI] [PubMed] [Google Scholar]
- Parry D. A. The molecular and fibrillar structure of collagen and its relationship to the mechanical properties of connective tissue. Biophys Chem. 1988 Feb;29(1-2):195–209. doi: 10.1016/0301-4622(88)87039-x. [DOI] [PubMed] [Google Scholar]
- Rowe R. W. The structure of rat tail tendon fascicles. Connect Tissue Res. 1985;14(1):21–30. doi: 10.3109/03008208509089840. [DOI] [PubMed] [Google Scholar]
- Sasaki N., Odajima S. Elongation mechanism of collagen fibrils and force-strain relations of tendon at each level of structural hierarchy. J Biomech. 1996 Sep;29(9):1131–1136. doi: 10.1016/0021-9290(96)00024-3. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Cummings C., Greiling H., Stuhlsatz H. W., Gregory J. D., Damle S. P. Examination of corneal proteoglycans and glycosaminoglycans by rotary shadowing and electron microscopy. Int J Biol Macromol. 1990 Jun;12(3):180–184. doi: 10.1016/0141-8130(90)90029-a. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Haigh M. Proteoglycan-type I collagen fibril interactions in bone and non-calcifying connective tissues. Biosci Rep. 1985 Jan;5(1):71–81. doi: 10.1007/BF01117443. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Orford C. R. Dermatan sulphate-rich proteoglycan associates with rat tail-tendon collagen at the d band in the gap region. Biochem J. 1981 Jul 1;197(1):213–216. doi: 10.1042/bj1970213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Parry D. A. Control of collagen fibril diameters in tissues. Int J Biol Macromol. 1992 Oct;14(5):292–293. doi: 10.1016/s0141-8130(05)80043-1. [DOI] [PubMed] [Google Scholar]
- Scott J. E. Proteodermatan and proteokeratan sulfate (decorin, lumican/fibromodulin) proteins are horseshoe shaped. Implications for their interactions with collagen. Biochemistry. 1996 Jul 9;35(27):8795–8799. doi: 10.1021/bi960773t. [DOI] [PubMed] [Google Scholar]
- Scott J. E. The periphery of the developing collagen fibril. Quantitative relationships with dermatan sulphate and other surface-associated species. Biochem J. 1984 Feb 15;218(1):229–233. doi: 10.1042/bj2180229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver F. H., Langley K. H., Trelstad R. L. Type I collagen fibrillogenesis: initiation via reversible linear and lateral growth steps. Biopolymers. 1979 Oct;18(10):2523–2535. doi: 10.1002/bip.1979.360181011. [DOI] [PubMed] [Google Scholar]
- Silver F. H., Trelstad R. L. Linear aggregation and the turbidimetric lag phase: type I collagen fibrillogenesis in vitro. J Theor Biol. 1979 Dec 7;81(3):515–526. doi: 10.1016/0022-5193(79)90049-3. [DOI] [PubMed] [Google Scholar]
- Silver F. H., Trelstad R. L. Type I collagen in solution. Structure and properties of fibril fragments. J Biol Chem. 1980 Oct 10;255(19):9427–9433. [PubMed] [Google Scholar]
- Staatz W. D., Fok K. F., Zutter M. M., Adams S. P., Rodriguez B. A., Santoro S. A. Identification of a tetrapeptide recognition sequence for the alpha 2 beta 1 integrin in collagen. J Biol Chem. 1991 Apr 25;266(12):7363–7367. [PubMed] [Google Scholar]
- Trelstad R. L., Hayashi K. Tendon collagen fibrillogenesis: intracellular subassemblies and cell surface changes associated with fibril growth. Dev Biol. 1979 Aug;71(2):228–242. doi: 10.1016/0012-1606(79)90166-0. [DOI] [PubMed] [Google Scholar]
- Vilarta R., Vidal B. de C. Anisotropic and biomechanical properties of tendons modified by exercise and denervation: aggregation and macromolecular order in collagen bundles. Matrix. 1989 Jan;9(1):55–61. doi: 10.1016/s0934-8832(89)80019-8. [DOI] [PubMed] [Google Scholar]
- Vogel K. G., Trotter J. A. The effect of proteoglycans on the morphology of collagen fibrils formed in vitro. Coll Relat Res. 1987 Jun;7(2):105–114. doi: 10.1016/s0174-173x(87)80002-x. [DOI] [PubMed] [Google Scholar]
- Wang M. C., Pins G. D., Silver F. H. Collagen fibres with improved strength for the repair of soft tissue injuries. Biomaterials. 1994 Jun;15(7):507–512. doi: 10.1016/0142-9612(94)90016-7. [DOI] [PubMed] [Google Scholar]
- Watts G. T., Grillo H. C., Gross J. Studies in Wound Healing: II. The Role of Granulation Tissue in Contraction. Ann Surg. 1958 Aug;148(2):153–160. doi: 10.1097/00000658-195808000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Campos Vidal B., de Carvalho H. F. Aggregational state and molecular order of tendons as a function of age. Matrix. 1990 Mar;10(1):48–57. doi: 10.1016/s0934-8832(11)80137-x. [DOI] [PubMed] [Google Scholar]