Abstract
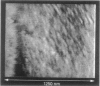
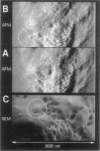
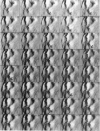
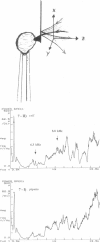
Single living cells were studied in growth medium by atomic force microscopy at a high--down to one image frame per second--imaging rate over time periods of many hours, stably producing hundreds of consecutive scans with a lateral resolution of approximately 30-40 nm. The cell was held by a micropipette mounted onto the scanner-piezo as shown in Häberle, W., J. K. H. Hörber, and G. Binnig. 1991. Force microscopy on living cells. J. Vac. Sci. Technol. B9:1210-0000. To initiate specific processes on the cell surface the cells had been infected with pox viruses as reported earlier and, most likely, the liberation of a progeny virion by the still-living cell was observed, hence confirming and supporting earlier results (Häberle, W., J. K. H. Hörber, F. Ohnesorge, D. P. E. Smith, and G. Binnig. 1992. In situ investigations of single living cells infected by viruses. Ultramicroscopy. 42-44:1161-0000; Hörber, J. K. H., W. Häberle, F. Ohnesorge, G. Binnig, H. G. Liebich, C. P. Czerny, H. Mahnel, and A. Mayr. 1992. Investigation of living cells in the nanometer regime with the atomic force microscope. Scanning Microscopy. 6:919-930). Furthermore, the pox viruses used were characterized separately by AFM in an aqueous environment down to the molecular level. Quasi-ordered structural details were resolved on a scale of a few nm where, however, image distortions and artifacts due to multiple tip effects are probably involved--just as in very high resolution (<15-20 nm) images on the cells. Although in a very preliminary manner, initial studies on the mechanical resonance properties of a single living (noninfected) cell, held by the micropipette, have been performed. In particular, frequency response spectra were recorded that indicate elastic properties and enough stiffness of these cells to make the demonstrated rapid scanning of the imaging tip plausible. Measurements of this kind, especially if they can be proven to be cell-type specific, may perhaps have a large potential for biomedical applications. Images of these living cells were also recorded in the widely known (e.g., Radmacher, M., R. W. Tillmann, and H. E. Gaub. 1993. Imaging viscoelasticity by force modulation with the atomic force microscope. Biophys. J. 64:735-742) force modulation mode, yet at one low modulation frequency of approximately 2 kHz. (Note: After the cells were attached to the pipette by suction, they first deformed significantly and then reassumed their original spherical shape, which they also acquire when freely suspended in solution, to a great extent with the exception of the portion adjusting to the pipette edge geometry after approximately 0.5-1 h, which occurred in almost the same manner with uninfected cells, and those that had been infected several hours earlier. This seems to be a process which is at least actively supported by the cellular cytoskeleton, rather than a mere osmotic pressure effect induced by electrolyte transport through the membrane. Furthermore, several hours postinfection (p.i.) infected cells developed many optically visible refraction effects, which appeared as small dark spots in the light microscope, that we believed to be the regions in the cell plasma where viruses are assembled; this is known from the literature on electron microscopy on pox-infected cells and referred to there as "virus factories" (e.g., Moss, B. 1986. Replication of pox viruses. In Fundamental Virology, B. N. Fields and D. M. Knape, editors. Raven Press, New York. 637-655). Therefore, we assume that the cells stay alive during imaging, in our experience for approximately 30-45 h p.i.).
Full text
PDF

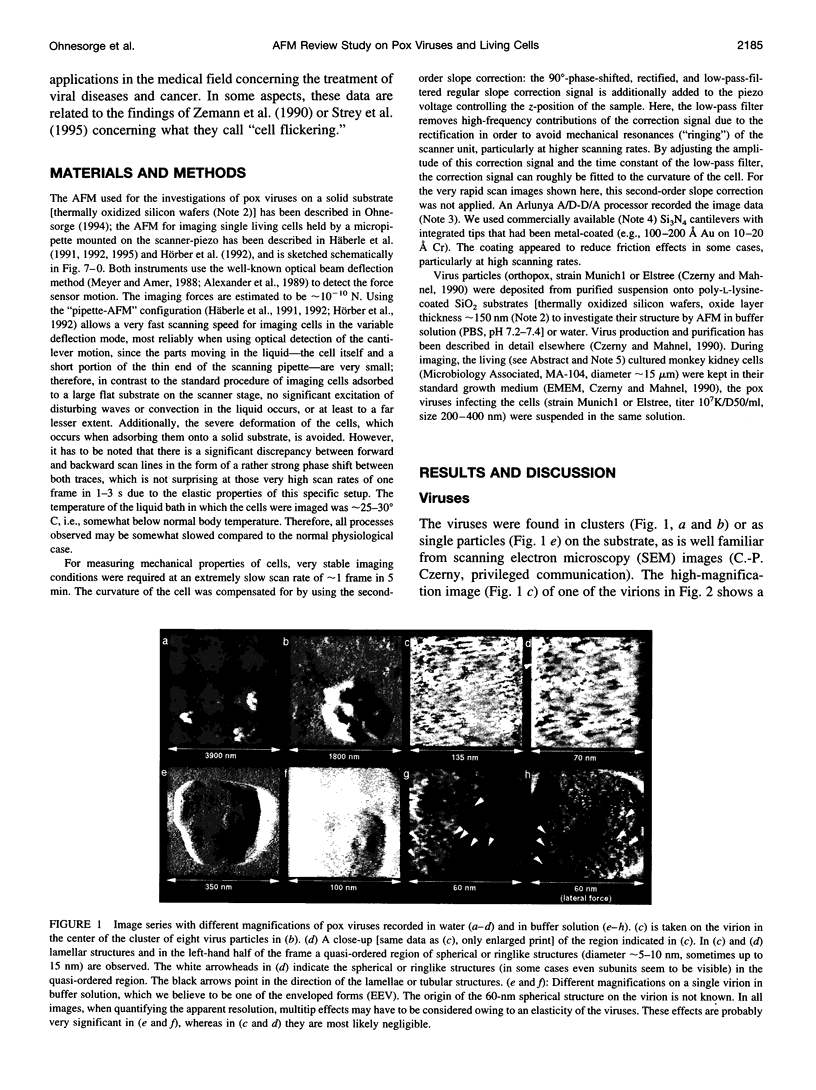



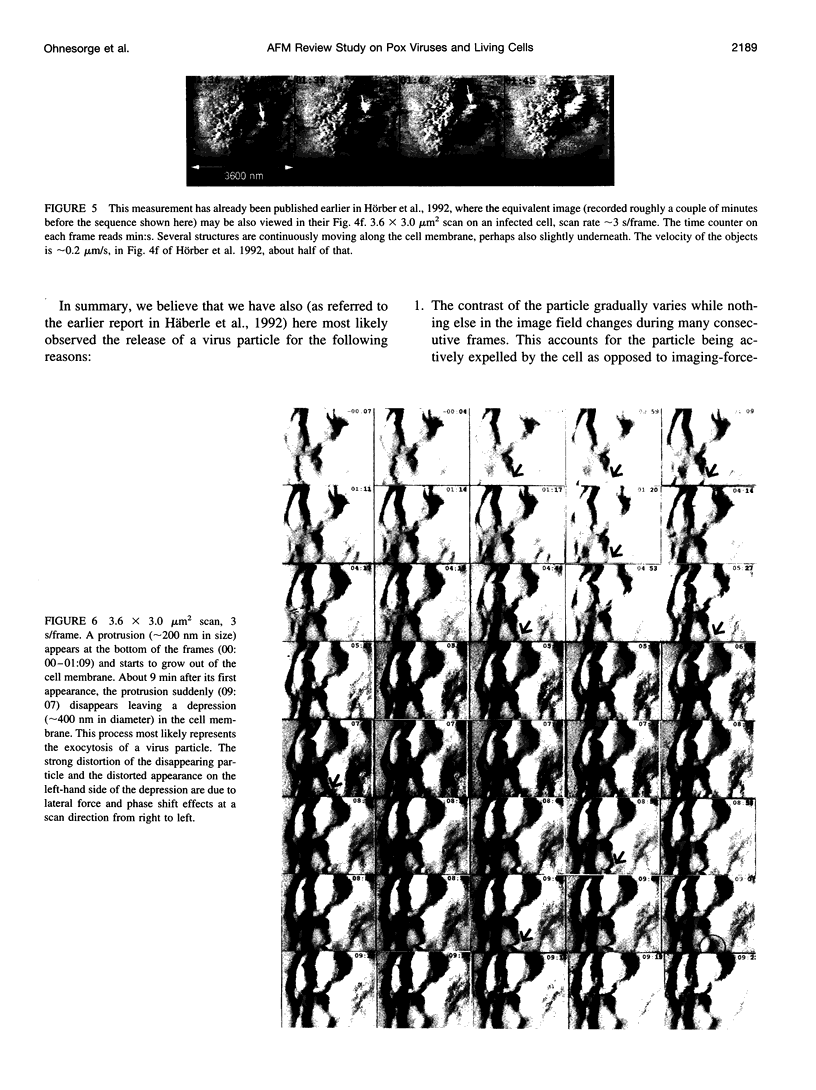
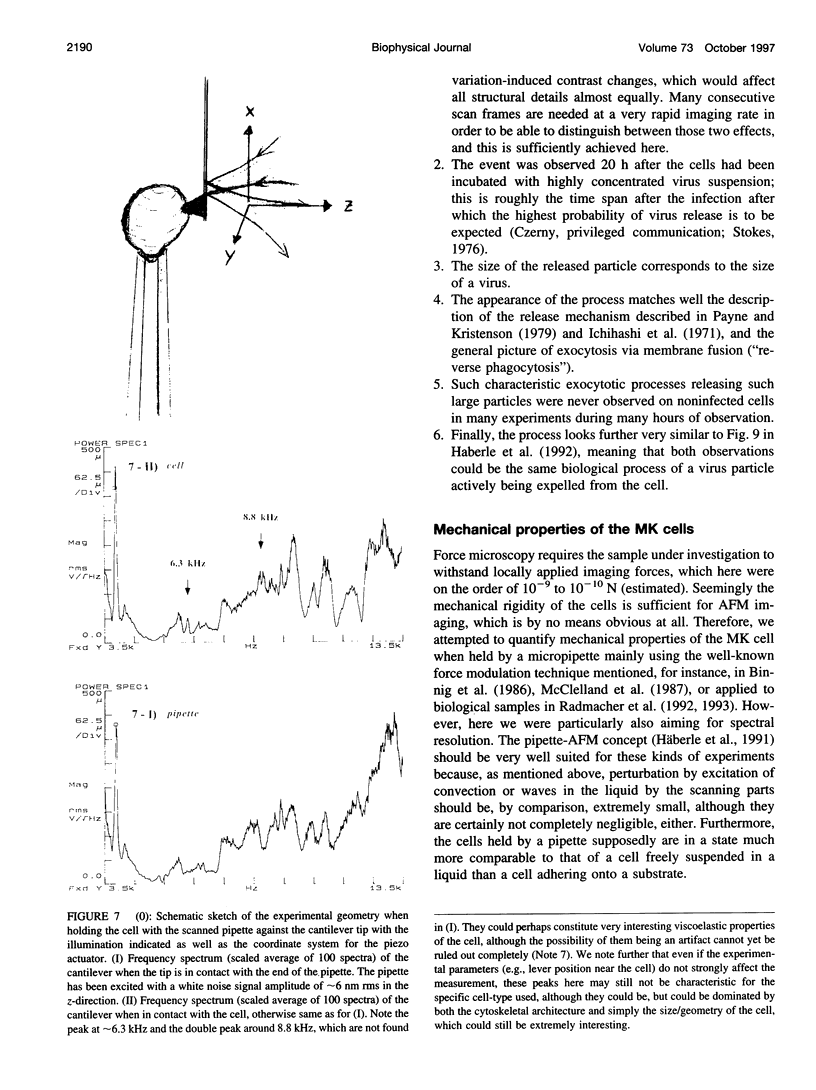




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binnig G, Quate CF, Gerber C. Atomic force microscope. Phys Rev Lett. 1986 Mar 3;56(9):930–933. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- Blasco R., Moss B. Extracellular vaccinia virus formation and cell-to-cell virus transmission are prevented by deletion of the gene encoding the 37,000-Dalton outer envelope protein. J Virol. 1991 Nov;65(11):5910–5920. doi: 10.1128/jvi.65.11.5910-5920.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasco R., Moss B. Role of cell-associated enveloped vaccinia virus in cell-to-cell spread. J Virol. 1992 Jul;66(7):4170–4179. doi: 10.1128/jvi.66.7.4170-4179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein D., Spudich A. Structure and activation dynamics of RBL-2H3 cells observed with scanning force microscopy. Biophys J. 1994 May;66(5):1717–1725. doi: 10.1016/S0006-3495(94)80964-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt H. J., Wolff E. K., Gould S. A., Dixon Northern B., Peterson C. M., Hansma P. K. Imaging cells with the atomic force microscope. J Struct Biol. 1990 Oct-Dec;105(1-3):54–61. doi: 10.1016/1047-8477(90)90098-w. [DOI] [PubMed] [Google Scholar]
- Cudmore S., Cossart P., Griffiths G., Way M. Actin-based motility of vaccinia virus. Nature. 1995 Dec 7;378(6557):636–638. doi: 10.1038/378636a0. [DOI] [PubMed] [Google Scholar]
- Czerny C. P., Johann S., Hölzle L., Meyer H. Epitope detection in the envelope of intracellular naked orthopox viruses and identification of encoding genes. Virology. 1994 May 1;200(2):764–777. doi: 10.1006/viro.1994.1240. [DOI] [PubMed] [Google Scholar]
- Czerny C. P., Mahnel H. Structural and functional analysis of orthopoxvirus epitopes with neutralizing monoclonal antibodies. J Gen Virol. 1990 Oct;71(Pt 10):2341–2352. doi: 10.1099/0022-1317-71-10-2341. [DOI] [PubMed] [Google Scholar]
- Doms R. W., Blumenthal R., Moss B. Fusion of intra- and extracellular forms of vaccinia virus with the cell membrane. J Virol. 1990 Oct;64(10):4884–4892. doi: 10.1128/jvi.64.10.4884-4892.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake B., Prater C. B., Weisenhorn A. L., Gould S. A., Albrecht T. R., Quate C. F., Cannell D. S., Hansma H. G., Hansma P. K. Imaging crystals, polymers, and processes in water with the atomic force microscope. Science. 1989 Mar 24;243(4898):1586–1589. doi: 10.1126/science.2928794. [DOI] [PubMed] [Google Scholar]
- Hansma H. G., Hoh J. H. Biomolecular imaging with the atomic force microscope. Annu Rev Biophys Biomol Struct. 1994;23:115–139. doi: 10.1146/annurev.bb.23.060194.000555. [DOI] [PubMed] [Google Scholar]
- Hoh J. H., Hansma P. K. Atomic force microscopy for high-resolution imaging in cell biology. Trends Cell Biol. 1992 Jul;2(7):208–213. doi: 10.1016/0962-8924(92)90248-l. [DOI] [PubMed] [Google Scholar]
- Hoh J. H., Lal R., John S. A., Revel J. P., Arnsdorf M. F. Atomic force microscopy and dissection of gap junctions. Science. 1991 Sep 20;253(5026):1405–1408. doi: 10.1126/science.1910206. [DOI] [PubMed] [Google Scholar]
- Hoh J. H., Schoenenberger C. A. Surface morphology and mechanical properties of MDCK monolayers by atomic force microscopy. J Cell Sci. 1994 May;107(Pt 5):1105–1114. doi: 10.1242/jcs.107.5.1105. [DOI] [PubMed] [Google Scholar]
- Häberle W., Hörber J. K., Ohnesorge F., Smith D. P., Binnig G. In situ investigations of single living cells infected by viruses. Ultramicroscopy. 1992 Jul;42-44(Pt B):1161–1167. doi: 10.1016/0304-3991(92)90418-j. [DOI] [PubMed] [Google Scholar]
- Hörber J. K., Häberle W., Ohnesorge F., Binnig G., Liebich H. G., Czerny C. P., Mahnel H., Mayr A. Investigation of living cells in the nanometer regime with the scanning force microscope. Scanning Microsc. 1992 Dec;6(4):919–930. [PubMed] [Google Scholar]
- Ichihashi Y., Matsumoto S., Dales S. Biogenesis of poxviruses: role of A-type inclusions and host cell membranes in virus dissemination. Virology. 1971 Dec;46(3):507–532. doi: 10.1016/0042-6822(71)90056-0. [DOI] [PubMed] [Google Scholar]
- Karrasch S., Dolder M., Schabert F., Ramsden J., Engel A. Covalent binding of biological samples to solid supports for scanning probe microscopy in buffer solution. Biophys J. 1993 Dec;65(6):2437–2446. doi: 10.1016/S0006-3495(93)81327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrasch S., Hegerl R., Hoh J. H., Baumeister W., Engel A. Atomic force microscopy produces faithful high-resolution images of protein surfaces in an aqueous environment. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):836–838. doi: 10.1073/pnas.91.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasas S., Gotzos V., Celio M. R. Observation of living cells using the atomic force microscope. Biophys J. 1993 Feb;64(2):539–544. doi: 10.1016/S0006-3495(93)81396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krempien U., Schneider L., Hiller G., Weber K., Katz E., Jungwirth C. Conditions for pox virus-specific microvilli formation studied during synchronized virus assembly. Virology. 1981 Sep;113(2):556–564. doi: 10.1016/0042-6822(81)90183-5. [DOI] [PubMed] [Google Scholar]
- Lal R., Drake B., Blumberg D., Saner D. R., Hansma P. K., Feinstein S. C. Imaging real-time neurite outgrowth and cytoskeletal reorganization with an atomic force microscope. Am J Physiol. 1995 Jul;269(1 Pt 1):C275–C285. doi: 10.1152/ajpcell.1995.269.1.C275. [DOI] [PubMed] [Google Scholar]
- Maa J. S., Rodriguez J. F., Esteban M. Structural and functional characterization of a cell surface binding protein of vaccinia virus. J Biol Chem. 1990 Jan 25;265(3):1569–1577. [PubMed] [Google Scholar]
- Medzon E. L., Bauer H. Structural features of vaccinia virus revealed by negative staining, sectioning, and freeze-etching. Virology. 1970 Apr;40(4):860–867. doi: 10.1016/0042-6822(70)90132-7. [DOI] [PubMed] [Google Scholar]
- Morgan C. Vaccinia virus reexamined: development and release. Virology. 1976 Aug;73(1):43–58. doi: 10.1016/0042-6822(76)90059-3. [DOI] [PubMed] [Google Scholar]
- Ohnesorge F., Heckl W. M., Häberle W., Pum D., Sara M., Schindler H., Schilcher K., Kiener A., Smith D. P., Sleytr U. B. Scanning force microscopy studies of the S-layers from Bacillus coagulans E38-66, Bacillus sphaericus CCM2177 and of an antibody binding process. Ultramicroscopy. 1992 Jul;42-44(Pt B):1236–1242. doi: 10.1016/0304-3991(92)90429-n. [DOI] [PubMed] [Google Scholar]
- Payne L. G., Kristenson K. Mechanism of vaccinia virus release and its specific inhibition by N1-isonicotinoyl-N2-3-methyl-4-chlorobenzoylhydrazine. J Virol. 1979 Nov;32(2):614–622. doi: 10.1128/jvi.32.2.614-622.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne L. G., Kristensson K. The effect of cytochalasin D and monensin on enveloped vaccinia virus release. Arch Virol. 1982;74(1):11–20. doi: 10.1007/BF01320778. [DOI] [PubMed] [Google Scholar]
- Radmacher M., Fritz M., Hansma H. G., Hansma P. K. Direct observation of enzyme activity with the atomic force microscope. Science. 1994 Sep 9;265(5178):1577–1579. doi: 10.1126/science.8079171. [DOI] [PubMed] [Google Scholar]
- Radmacher M., Tillamnn R. W., Fritz M., Gaub H. E. From molecules to cells: imaging soft samples with the atomic force microscope. Science. 1992 Sep 25;257(5078):1900–1905. doi: 10.1126/science.1411505. [DOI] [PubMed] [Google Scholar]
- Radmacher M., Tillmann R. W., Gaub H. E. Imaging viscoelasticity by force modulation with the atomic force microscope. Biophys J. 1993 Mar;64(3):735–742. doi: 10.1016/S0006-3495(93)81433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. F., Paez E., Esteban M. A 14,000-Mr envelope protein of vaccinia virus is involved in cell fusion and forms covalently linked trimers. J Virol. 1987 Feb;61(2):395–404. doi: 10.1128/jvi.61.2.395-404.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabert F. A., Henn C., Engel A. Native Escherichia coli OmpF porin surfaces probed by atomic force microscopy. Science. 1995 Apr 7;268(5207):92–94. doi: 10.1126/science.7701347. [DOI] [PubMed] [Google Scholar]
- Schoenenberger C. A., Hoh J. H. Slow cellular dynamics in MDCK and R5 cells monitored by time-lapse atomic force microscopy. Biophys J. 1994 Aug;67(2):929–936. doi: 10.1016/S0006-3495(94)80556-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroff S. G., Saner D. R., Lal R. Dynamic micromechanical properties of cultured rat atrial myocytes measured by atomic force microscopy. Am J Physiol. 1995 Jul;269(1 Pt 1):C286–C292. doi: 10.1152/ajpcell.1995.269.1.C286. [DOI] [PubMed] [Google Scholar]
- Sodeik B., Griffiths G., Ericsson M., Moss B., Doms R. W. Assembly of vaccinia virus: effects of rifampin on the intracellular distribution of viral protein p65. J Virol. 1994 Feb;68(2):1103–1114. doi: 10.1128/jvi.68.2.1103-1114.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich A., Braunstein D. Large secretory structures at the cell surface imaged with scanning force microscopy. Proc Natl Acad Sci U S A. 1995 Jul 18;92(15):6976–6980. doi: 10.1073/pnas.92.15.6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern W., Dales S. Biogenesis of vaccinia: isolation and characterization of a surface component that elicits antibody suppressing infectivity and cell-cell fusion. Virology. 1976 Nov;75(1):232–241. doi: 10.1016/0042-6822(76)90022-2. [DOI] [PubMed] [Google Scholar]
- Stokes G. V. High-voltage electron microscope study of the release of vaccinia virus from whole cells. J Virol. 1976 May;18(2):636–643. doi: 10.1128/jvi.18.2.636-643.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltz D. B., Summers M. D. Observations on the morphogenesis and structure of a hemocytic poxvirus in the midge Chironomus attenuatus. J Ultrastruct Res. 1972 Sep;40(5):581–598. doi: 10.1016/s0022-5320(72)80045-5. [DOI] [PubMed] [Google Scholar]
- Strey H., Peterson M., Sackmann E. Measurement of erythrocyte membrane elasticity by flicker eigenmode decomposition. Biophys J. 1995 Aug;69(2):478–488. doi: 10.1016/S0006-3495(95)79921-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe W. P., Toner M., Ezzell R. M., Tompkins R. G., Yarmush M. L. Dynamics of photoinduced cell plasma membrane injury. Biophys J. 1995 May;68(5):2198–2206. doi: 10.1016/S0006-3495(95)80402-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui K., Uno F., Akatsuka K., Nii S. Electron microscopic study on vaccinia virus release. Arch Virol. 1983;75(3):213–218. doi: 10.1007/BF01315275. [DOI] [PubMed] [Google Scholar]
- Yang J., Tamm L. K., Tillack T. W., Shao Z. New approach for atomic force microscopy of membrane proteins. The imaging of cholera toxin. J Mol Biol. 1993 Jan 20;229(2):286–290. doi: 10.1006/jmbi.1993.1033. [DOI] [PubMed] [Google Scholar]
- Zeman K., Engelhard H., Sackmann E. Bending undulations and elasticity of the erythrocyte membrane: effects of cell shape and membrane organization. Eur Biophys J. 1990;18(4):203–219. doi: 10.1007/BF00183373. [DOI] [PubMed] [Google Scholar]