Abstract
A series of homologous amphiphilic molecules with surface areas in the range of 0.3 nm2 to 3.0 nm2 were prepared and used to investigate the diffusion in model dimyristoylphosphatidylcholine membranes as a function of temperature. The diffusion behavior of smaller molecules can be described by the interfacial viscosity limited free area theory promoted by Vaz and his co-workers, and that of the larger molecules can best be modeled by a recent interpretation of the theoretical description proposed by Evans and Sackmann. The experimental data show that the rate of diffusion is controlled by the size of the molecules at the interface of the lipid membrane, and provide evidence for a view of the membrane as a hydrodynamic triple layer with a low-viscosity central layer encased by two more viscous, yet fluid, layers.
Full text
PDF
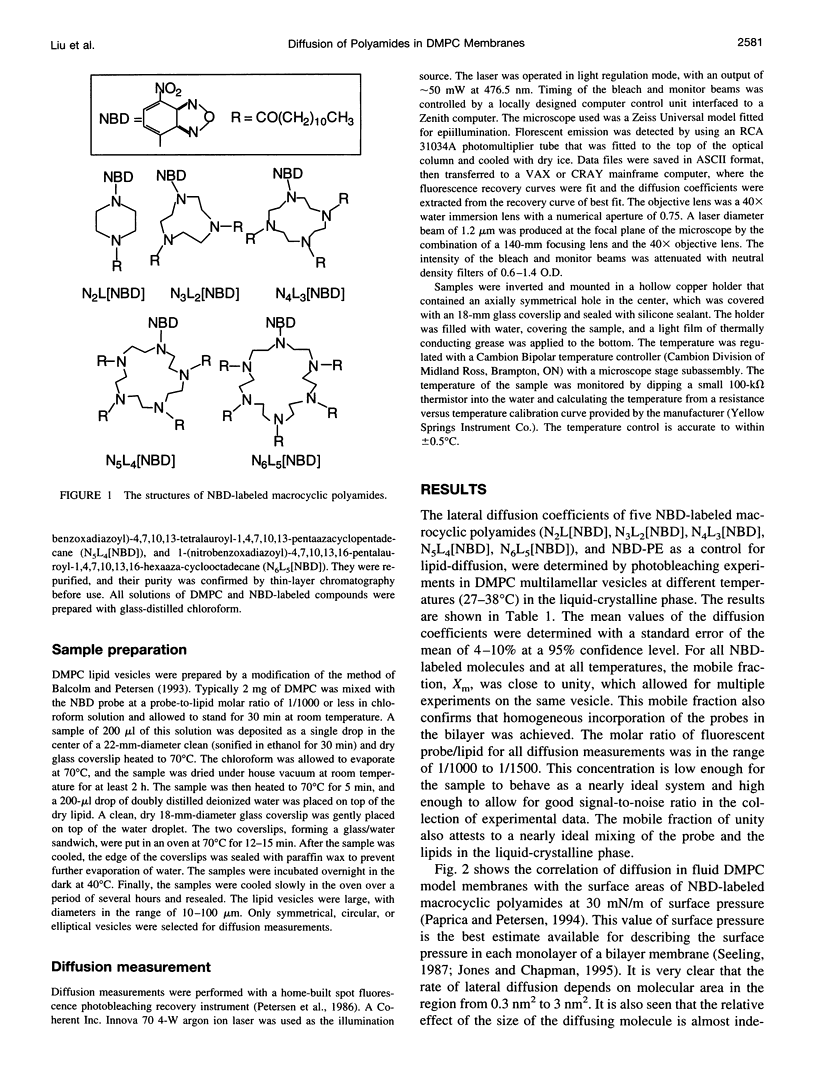
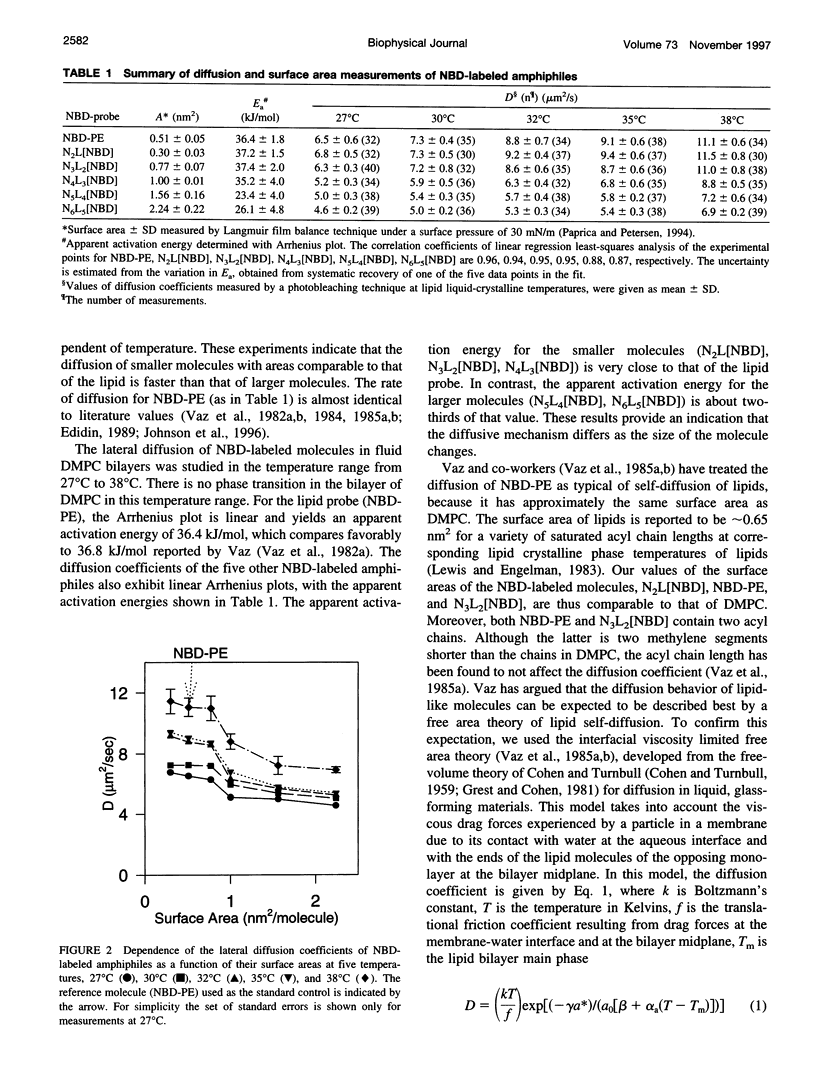

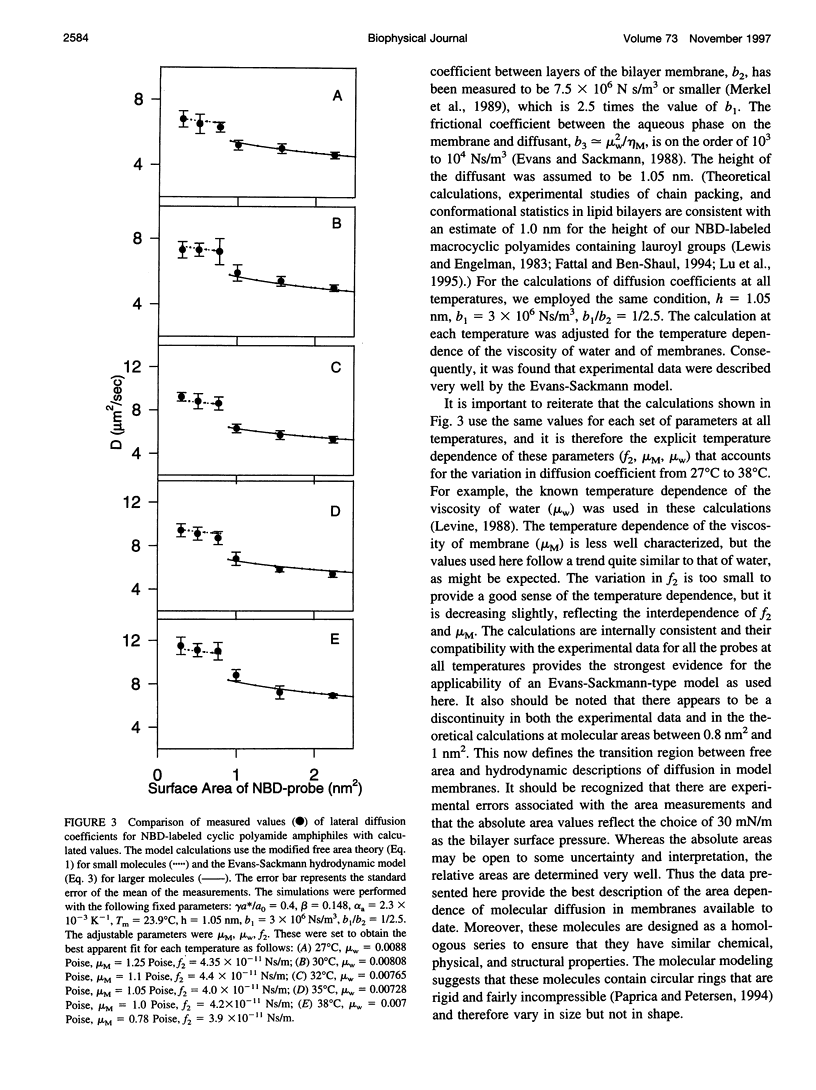
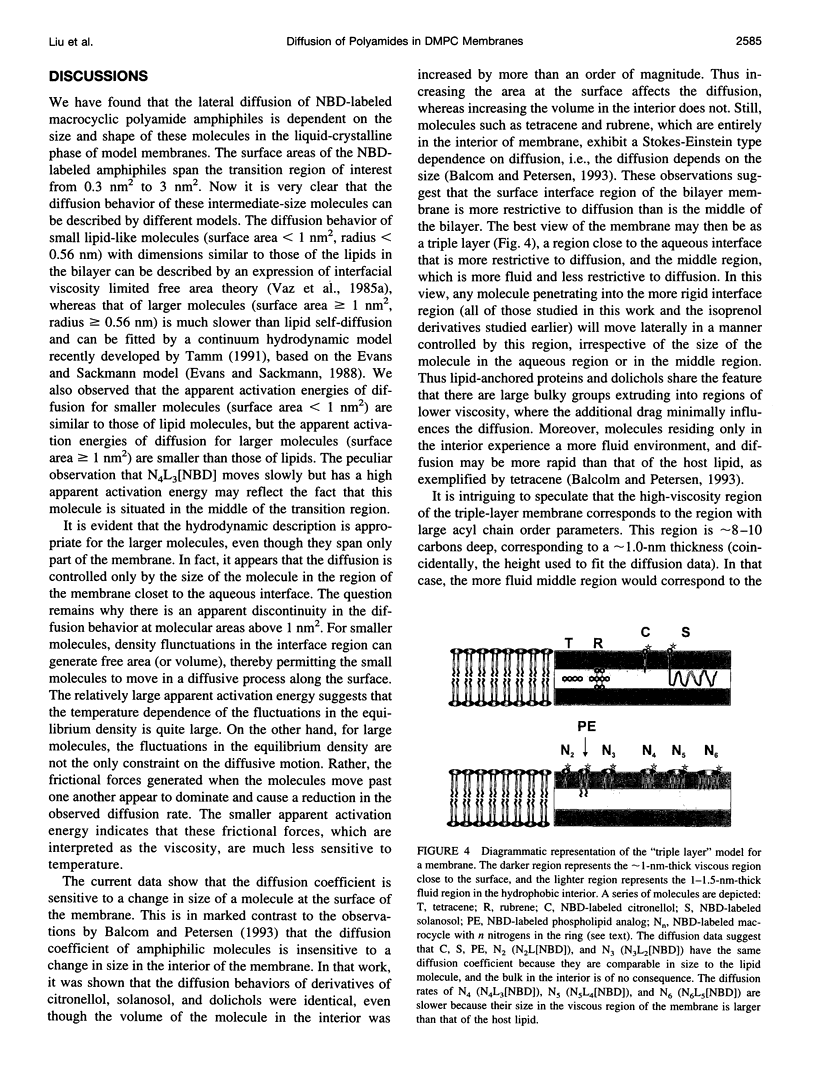


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcom B. J., Petersen N. O. Lateral diffusion in model membranes is independent of the size of the hydrophobic region of molecules. Biophys J. 1993 Aug;65(2):630–637. doi: 10.1016/S0006-3495(93)81106-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry R. J., Godfrey R. E. Anisotropic rotation of bacteriorhodopsin in lipid membranes. Comparison of theory with experiment. Biophys J. 1981 Oct;36(1):257–276. doi: 10.1016/S0006-3495(81)84727-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derzko Z., Jacobson K. Comparative lateral diffusion of fluorescent lipid analogues in phospholipid multibilayers. Biochemistry. 1980 Dec 23;19(26):6050–6057. doi: 10.1021/bi00567a016. [DOI] [PubMed] [Google Scholar]
- Edidin M., Zagyansky Y., Lardner T. J. Measurement of membrane protein lateral diffusion in single cells. Science. 1976 Feb 6;191(4226):466–468. doi: 10.1126/science.1246629. [DOI] [PubMed] [Google Scholar]
- Fattal D. R., Ben-Shaul A. Mean-field calculations of chain packing and conformational statistics in lipid bilayers: comparison with experiments and molecular dynamics studies. Biophys J. 1994 Sep;67(3):985–995. [PMC free article] [PubMed] [Google Scholar]
- Frey S., Tamm L. K. Membrane insertion and lateral diffusion of fluorescence-labelled cytochrome c oxidase subunit IV signal peptide in charged and uncharged phospholipid bilayers. Biochem J. 1990 Dec 15;272(3):713–719. doi: 10.1042/bj2720713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galla H. J., Hartmann W., Theilen U., Sackmann E. On two-dimensional passive random walk in lipid bilayers and fluid pathways in biomembranes. J Membr Biol. 1979 Jul 31;48(3):215–236. doi: 10.1007/BF01872892. [DOI] [PubMed] [Google Scholar]
- Hildebrand J. H. Motions of molecules in liquids: viscosity and diffusivity. Science. 1971 Oct 29;174(4008):490–493. doi: 10.1126/science.174.4008.490. [DOI] [PubMed] [Google Scholar]
- Hughes B. D., Pailthorpe B. A., White L. R., Sawyer W. H. Extraction of membrane microviscosity from translational and rotational diffusion coefficients. Biophys J. 1982 Mar;37(3):673–676. [PMC free article] [PubMed] [Google Scholar]
- Johnson M. E., Berk D. A., Blankschtein D., Golan D. E., Jain R. K., Langer R. S. Lateral diffusion of small compounds in human stratum corneum and model lipid bilayer systems. Biophys J. 1996 Nov;71(5):2656–2668. doi: 10.1016/S0006-3495(96)79457-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B. A., Engelman D. M. Lipid bilayer thickness varies linearly with acyl chain length in fluid phosphatidylcholine vesicles. J Mol Biol. 1983 May 15;166(2):211–217. doi: 10.1016/s0022-2836(83)80007-2. [DOI] [PubMed] [Google Scholar]
- Lu D., Vavasour I., Morrow M. R. Smoothed acyl chain orientational order parameter profiles in dimyristoylphosphatidylcholine-distearoylphosphatidylcholine mixtures: a 2H-NMR study. Biophys J. 1995 Feb;68(2):574–583. doi: 10.1016/S0006-3495(95)80219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R., Cherry R. J. Lateral and rotational diffusion of bacteriorhodopsin in lipid bilayers: experimental test of the Saffman-Delbrück equations. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4317–4321. doi: 10.1073/pnas.79.14.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackmann E. Supported membranes: scientific and practical applications. Science. 1996 Jan 5;271(5245):43–48. doi: 10.1126/science.271.5245.43. [DOI] [PubMed] [Google Scholar]
- Saffman P. G., Delbrück M. Brownian motion in biological membranes. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3111–3113. doi: 10.1073/pnas.72.8.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig A. Local anesthetics and pressure: a comparison of dibucaine binding to lipid monolayers and bilayers. Biochim Biophys Acta. 1987 May 29;899(2):196–204. doi: 10.1016/0005-2736(87)90400-7. [DOI] [PubMed] [Google Scholar]
- Tamm L. K. Membrane insertion and lateral mobility of synthetic amphiphilic signal peptides in lipid model membranes. Biochim Biophys Acta. 1991 Jul 22;1071(2):123–148. doi: 10.1016/0304-4157(91)90021-n. [DOI] [PubMed] [Google Scholar]
- Vaz W. L., Clegg R. M., Hallmann D. Translational diffusion of lipids in liquid crystalline phase phosphatidylcholine multibilayers. A comparison of experiment with theory. Biochemistry. 1985 Jan 29;24(3):781–786. doi: 10.1021/bi00324a037. [DOI] [PubMed] [Google Scholar]
- Vaz W. L., Criado M., Madeira V. M., Schoellmann G., Jovin T. M. Size dependence of the translational diffusion of large integral membrane proteins in liquid-crystalline phase lipid bilayers. A study using fluorescence recovery after photobleaching. Biochemistry. 1982 Oct 26;21(22):5608–5612. doi: 10.1021/bi00265a034. [DOI] [PubMed] [Google Scholar]
- Vaz W. L., Hallmann D., Clegg R. M., Gambacorta A., De Rosa M. A comparison of the translational diffusion of a normal and a membrane-spanning lipid in L alpha phase 1-palmitoyl-2-oleoylphosphatidylcholine bilayers. Eur Biophys J. 1985;12(1):19–24. doi: 10.1007/BF00254091. [DOI] [PubMed] [Google Scholar]



