Abstract
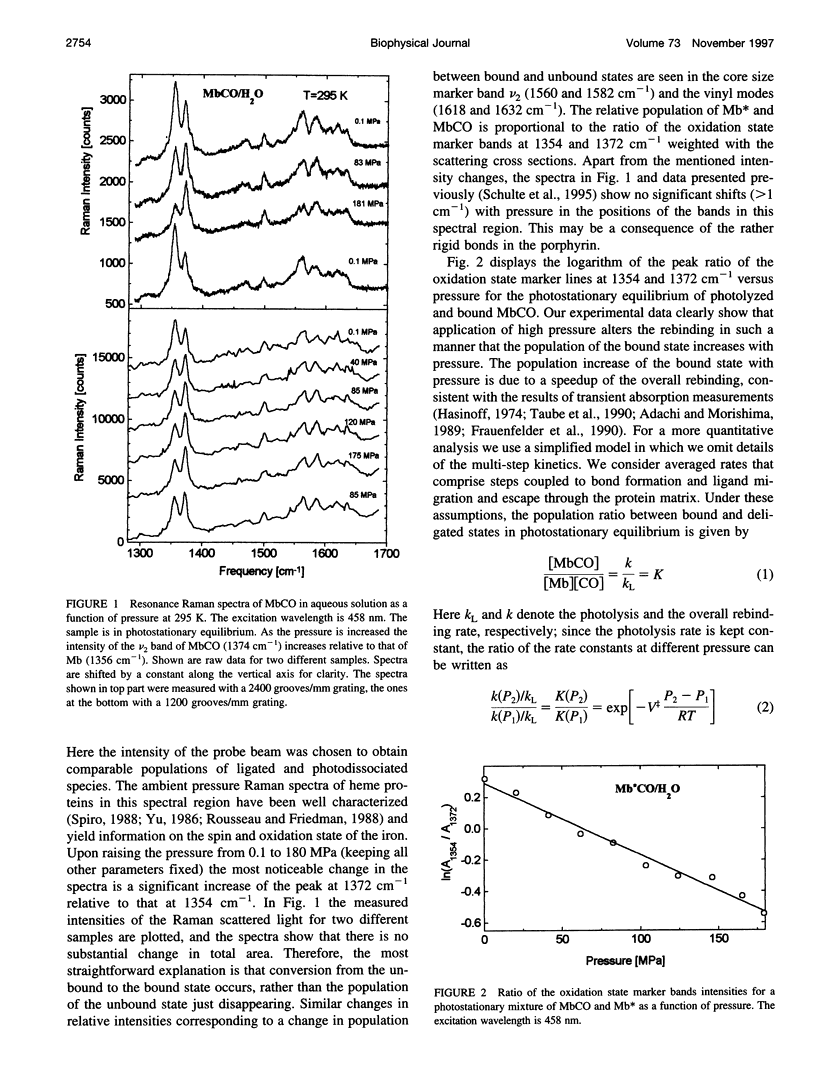
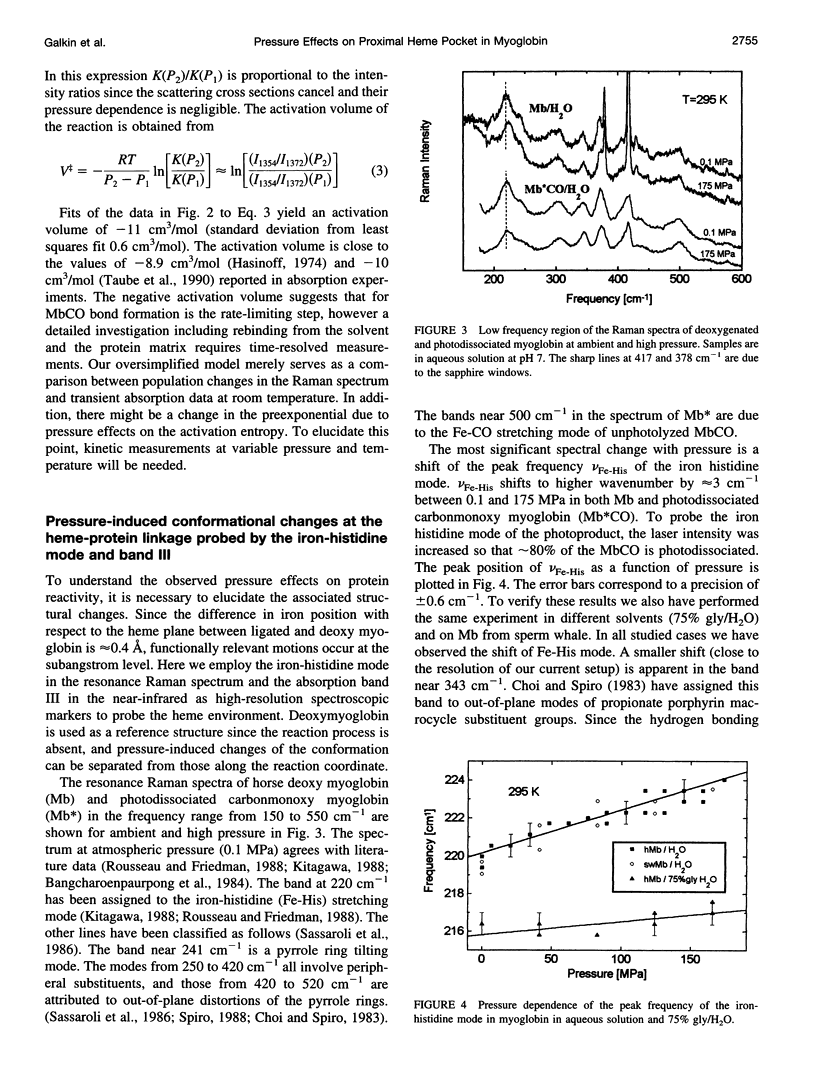
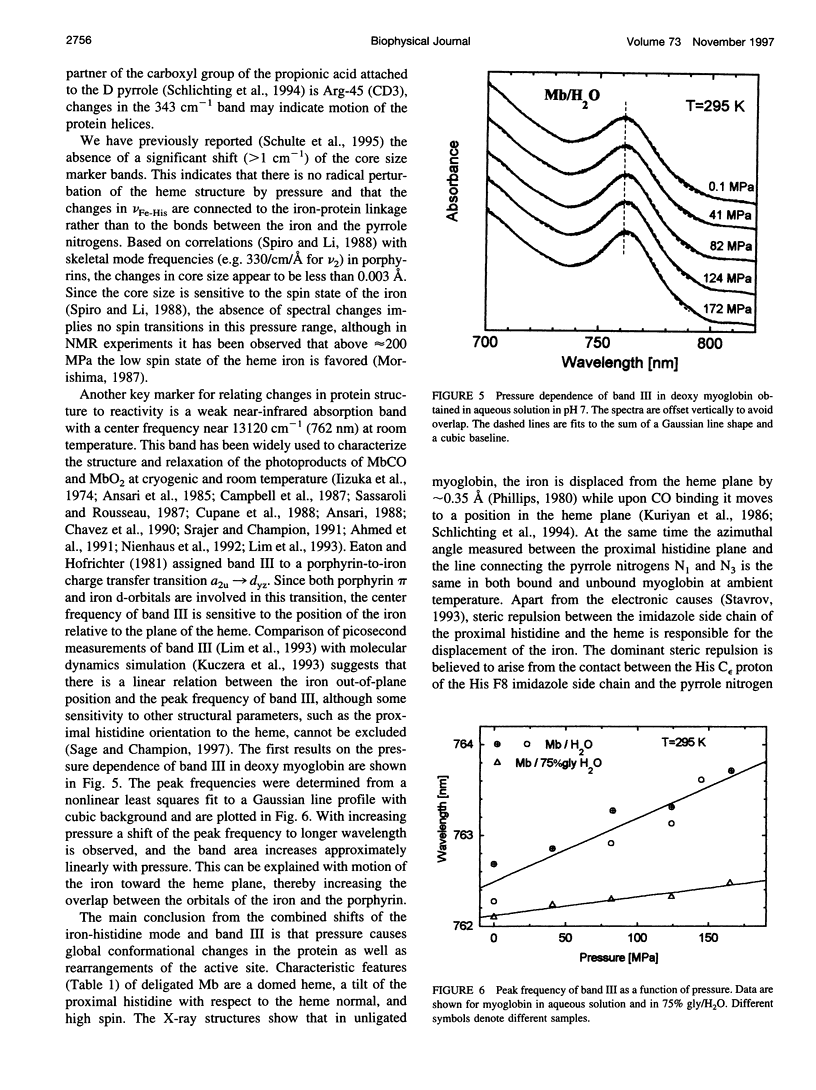
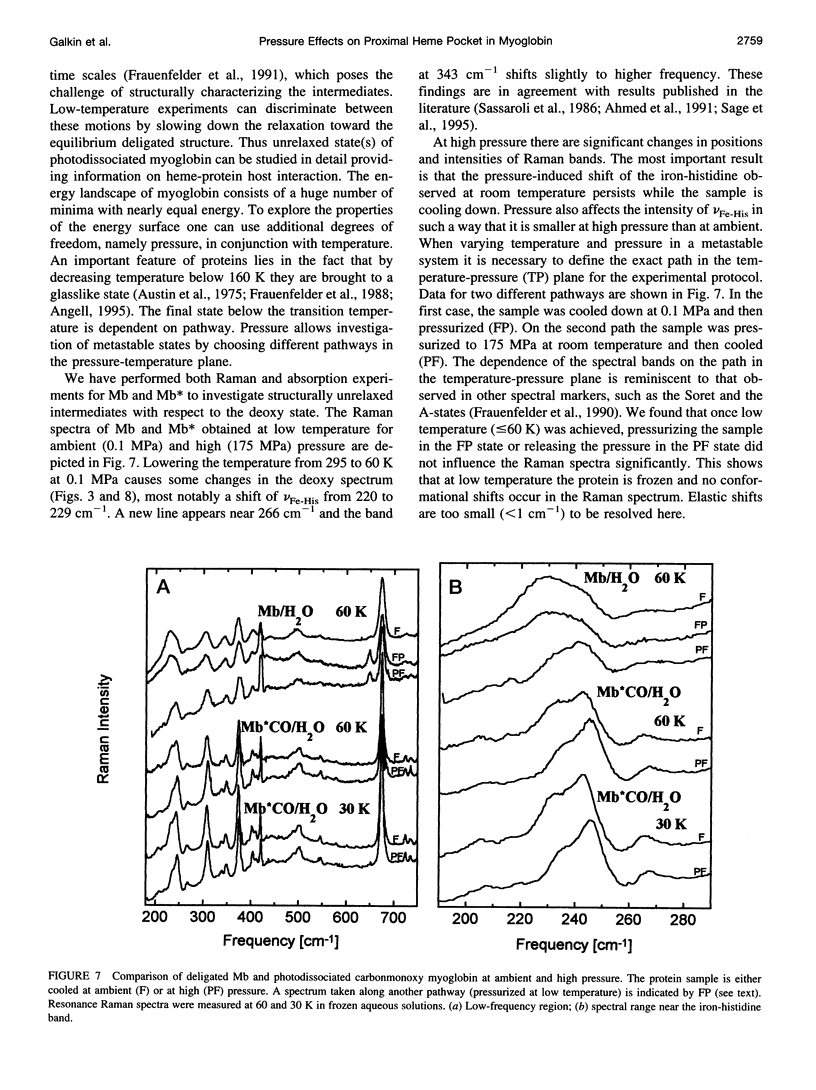
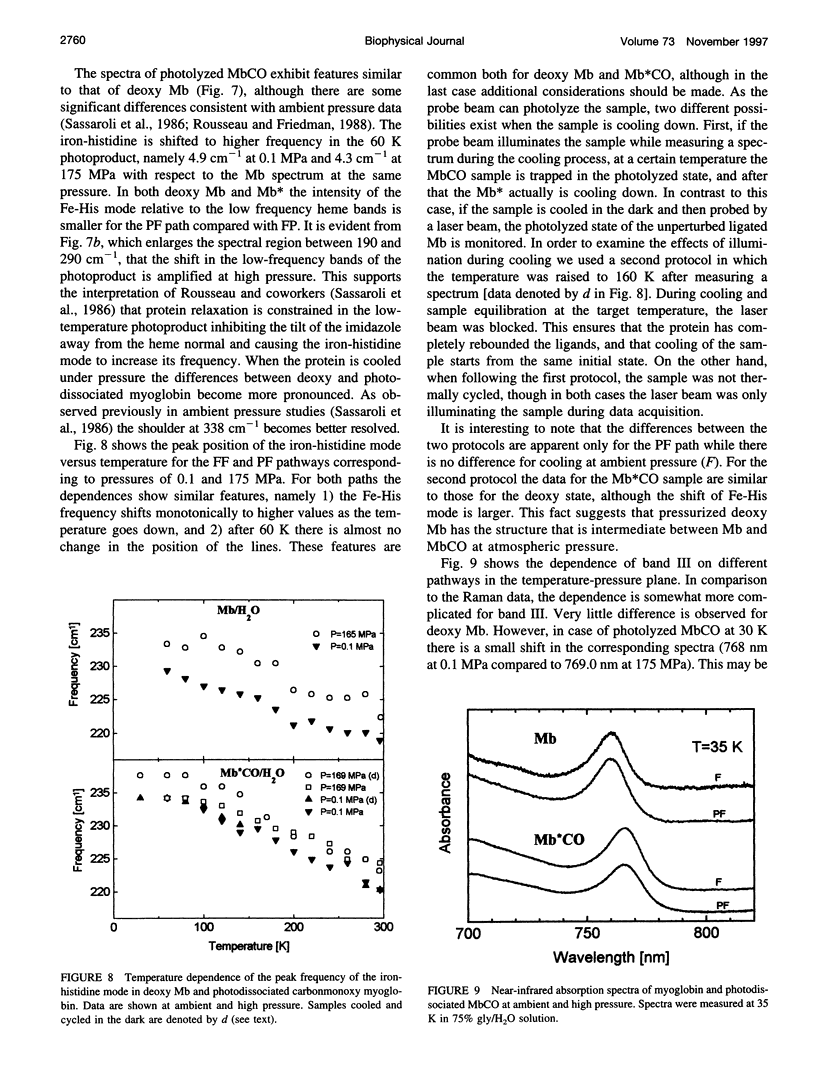
The influence of high pressure on the heme protein conformation of myoglobin in different ligation states is studied using Raman spectroscopy over the temperature range from 30 to 295 K. Photostationary experiments monitoring the oxidation state marker bands demonstrate the change of rebinding rate with pressure. While frequency changes of vibrational modes associated with rigid bonds of the porphyrin ring are <1 cm(-1), we investigate a significant shift of the iron-histidine mode to higher frequency with increasing pressure (approximately 3 cm(-1) for deltaP = 190 MPa in Mb). The observed frequency shift is interpreted structurally as a conformational change affecting the tilt angle between the heme plane and the proximal histidine and the out-of-plane iron position. Independent evidence for iron motion comes from measurements of the redshift of band III in the near-infrared with pressure. This suggests that at high pressure the proximal heme pocket and the protein are altered toward the bound state conformation, which contributes to the rate increase for CO binding. Raman spectra of Mb and photodissociated MbCO measured at low temperature and variable pressure further support changes in protein conformation and are consistent with glasslike properties of myoglobin below 160 K.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi S., Morishima I. The effects of pressure on oxygen and carbon monoxide binding kinetics for myoglobin. A high pressure laser flash photolysis study. J Biol Chem. 1989 Nov 15;264(32):18896–18901. [PubMed] [Google Scholar]
- Angell C. A. Formation of glasses from liquids and biopolymers. Science. 1995 Mar 31;267(5206):1924–1935. doi: 10.1126/science.267.5206.1924. [DOI] [PubMed] [Google Scholar]
- Ansari A., Berendzen J., Bowne S. F., Frauenfelder H., Iben I. E., Sauke T. B., Shyamsunder E., Young R. D. Protein states and proteinquakes. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5000–5004. doi: 10.1073/pnas.82.15.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari A., Berendzen J., Braunstein D., Cowen B. R., Frauenfelder H., Hong M. K., Iben I. E., Johnson J. B., Ormos P., Sauke T. B. Rebinding and relaxation in the myoglobin pocket. Biophys Chem. 1987 May 9;26(2-3):337–355. doi: 10.1016/0301-4622(87)80034-0. [DOI] [PubMed] [Google Scholar]
- Austin R. H., Beeson K. W., Eisenstein L., Frauenfelder H., Gunsalus I. C. Dynamics of ligand binding to myoglobin. Biochemistry. 1975 Dec 2;14(24):5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- Balasubramanian S., Lambright D. G., Marden M. C., Boxer S. G. CO recombination to human myoglobin mutants in glycerol-water solutions. Biochemistry. 1993 Mar 9;32(9):2202–2212. doi: 10.1021/bi00060a011. [DOI] [PubMed] [Google Scholar]
- Campbell B. F., Chance M. R., Friedman J. M. Ligand binding channels reflected in the resonance Raman spectra of cryogenically trapped species of myoglobin. J Biol Chem. 1987 Nov 5;262(31):14885–14890. [PubMed] [Google Scholar]
- Chavez M. D., Courtney S. H., Chance M. R., Kiula D., Nocek J., Hoffman B. M., Friedman J. M., Ondrias M. R. Structural and functional significance of inhomogeneous line broadening of band III in hemoglobin and Fe-Mn hybrid hemoglobins. Biochemistry. 1990 May 22;29(20):4844–4852. doi: 10.1021/bi00472a014. [DOI] [PubMed] [Google Scholar]
- Cheng X. D., Schoenborn B. P. Neutron diffraction study of carbonmonoxymyoglobin. J Mol Biol. 1991 Jul 20;220(2):381–399. doi: 10.1016/0022-2836(91)90020-7. [DOI] [PubMed] [Google Scholar]
- Cupane A., Leone M., Vitrano E., Cordone L. Structural and dynamic properties of the heme pocket in myoglobin probed by optical spectroscopy. Biopolymers. 1988 Dec;27(12):1977–1997. doi: 10.1002/bip.360271209. [DOI] [PubMed] [Google Scholar]
- Doster W., Cusack S., Petry W. Dynamical transition of myoglobin revealed by inelastic neutron scattering. Nature. 1989 Feb 23;337(6209):754–756. doi: 10.1038/337754a0. [DOI] [PubMed] [Google Scholar]
- Eaton W. A., Hofrichter J. Polarized absorption and linear dichroism spectroscopy of hemoglobin. Methods Enzymol. 1981;76:175–261. doi: 10.1016/0076-6879(81)76126-3. [DOI] [PubMed] [Google Scholar]
- Elber R., Karplus M. Multiple conformational states of proteins: a molecular dynamics analysis of myoglobin. Science. 1987 Jan 16;235(4786):318–321. doi: 10.1126/science.3798113. [DOI] [PubMed] [Google Scholar]
- Fersht A., Winter G. Protein engineering. Trends Biochem Sci. 1992 Aug;17(8):292–295. doi: 10.1016/0968-0004(92)90438-f. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Parak F., Young R. D. Conformational substates in proteins. Annu Rev Biophys Biophys Chem. 1988;17:451–479. doi: 10.1146/annurev.bb.17.060188.002315. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Sligar S. G., Wolynes P. G. The energy landscapes and motions of proteins. Science. 1991 Dec 13;254(5038):1598–1603. doi: 10.1126/science.1749933. [DOI] [PubMed] [Google Scholar]
- Friedman J. M., Campbell B. F., Noble R. W. A possible new control mechanism suggested by resonance Raman spectra from a deep ocean fish hemoglobin. Biophys Chem. 1990 Aug 31;37(1-3):43–59. doi: 10.1016/0301-4622(90)88006-e. [DOI] [PubMed] [Google Scholar]
- Friedman J. M. Structure, dynamics, and reactivity in hemoglobin. Science. 1985 Jun 14;228(4705):1273–1280. doi: 10.1126/science.4001941. [DOI] [PubMed] [Google Scholar]
- Gilch H., Dreybrodt W., Schweitzer-Stenner R. Thermal fluctuations between conformational substates of the Fe(2+)-HisF8 linkage in deoxymyoglobin probed by the Raman active Fe-N epsilon (HisF8) stretching vibration. Biophys J. 1995 Jul;69(1):214–227. doi: 10.1016/S0006-3495(95)79893-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilch H., Schweitzer-Stenner R., Dreybrodt W. Structural heterogeneity of the Fe(2+)-N epsilon (HisF8) bond in various hemoglobin and myoglobin derivatives probed by the Raman-active iron histidine stretching mode. Biophys J. 1993 Oct;65(4):1470–1485. doi: 10.1016/S0006-3495(93)81216-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasinoff B. B. Kinetic activation volumes of the binding of oxygen and carbon monoxide to hemoglobin and myoglobin studied on a high-pressure laser flash photolysis apparatus. Biochemistry. 1974 Jul 16;13(15):3111–3117. doi: 10.1021/bi00712a017. [DOI] [PubMed] [Google Scholar]
- Heremans K. High pressure effects on proteins and other biomolecules. Annu Rev Biophys Bioeng. 1982;11:1–21. doi: 10.1146/annurev.bb.11.060182.000245. [DOI] [PubMed] [Google Scholar]
- Iizuka T., Yamamoto H., Kotani M., Yonetani T. Low temperature photodissociation of hemoproteins: carbon monoxide complex of myoglobin and hemoglobin. Biochim Biophys Acta. 1974 Nov 5;371(1):126–139. doi: 10.1016/0005-2795(74)90161-5. [DOI] [PubMed] [Google Scholar]
- Jonas J., Jonas A. High-pressure NMR spectroscopy of proteins and membranes. Annu Rev Biophys Biomol Struct. 1994;23:287–318. doi: 10.1146/annurev.bb.23.060194.001443. [DOI] [PubMed] [Google Scholar]
- Kiger L., Stetzkowski-Marden F., Poyart C., Marden M. C. Correlation of carbon monoxide association rates and the position of absorption band III in hemoproteins. Eur J Biochem. 1995 Mar 15;228(3):665–668. doi: 10.1111/j.1432-1033.1995.tb20307.x. [DOI] [PubMed] [Google Scholar]
- Kitchen D. B., Reed L. H., Levy R. M. Molecular dynamics simulation of solvated protein at high pressure. Biochemistry. 1992 Oct 20;31(41):10083–10093. doi: 10.1021/bi00156a031. [DOI] [PubMed] [Google Scholar]
- Kuczera K., Lambry J. C., Martin J. L., Karplus M. Nonexponential relaxation after ligand dissociation from myoglobin: a molecular dynamics simulation. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5805–5807. doi: 10.1073/pnas.90.12.5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyan J., Wilz S., Karplus M., Petsko G. A. X-ray structure and refinement of carbon-monoxy (Fe II)-myoglobin at 1.5 A resolution. J Mol Biol. 1986 Nov 5;192(1):133–154. doi: 10.1016/0022-2836(86)90470-5. [DOI] [PubMed] [Google Scholar]
- Leeson D. T., Wiersma D. A. Looking into the energy landscape of myoglobin. Nat Struct Biol. 1995 Oct;2(10):848–851. doi: 10.1038/nsb1095-848. [DOI] [PubMed] [Google Scholar]
- Lim M., Jackson T. A., Anfinrud P. A. Nonexponential protein relaxation: dynamics of conformational change in myoglobin. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5801–5804. doi: 10.1073/pnas.90.12.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loncharich R. J., Brooks B. R. Temperature dependence of dynamics of hydrated myoglobin. Comparison of force field calculations with neutron scattering data. J Mol Biol. 1990 Oct 5;215(3):439–455. doi: 10.1016/s0022-2836(05)80363-8. [DOI] [PubMed] [Google Scholar]
- Morild E. The theory of pressure effects on enzymes. Adv Protein Chem. 1981;34:93–166. doi: 10.1016/s0065-3233(08)60519-7. [DOI] [PubMed] [Google Scholar]
- Nienhaus G. U., Mourant J. R., Chu K., Frauenfelder H. Ligand binding to heme proteins: the effect of light on ligand binding in myoglobin. Biochemistry. 1994 Nov 15;33(45):13413–13430. doi: 10.1021/bi00249a030. [DOI] [PubMed] [Google Scholar]
- Nienhaus G. U., Mourant J. R., Frauenfelder H. Spectroscopic evidence for conformational relaxation in myoglobin. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2902–2906. doi: 10.1073/pnas.89.7.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips S. E. Structure and refinement of oxymyoglobin at 1.6 A resolution. J Mol Biol. 1980 Oct 5;142(4):531–554. doi: 10.1016/0022-2836(80)90262-4. [DOI] [PubMed] [Google Scholar]
- Sassaroli M., Dasgupta S., Rousseau D. L. Cryogenic stabilization of myoglobin photoproducts. J Biol Chem. 1986 Oct 15;261(29):13704–13713. [PubMed] [Google Scholar]
- Sassaroli M., Rousseau D. L. Time dependence of near-infrared spectra of photodissociated hemoglobin and myoglobin. Biochemistry. 1987 Jun 2;26(11):3092–3098. doi: 10.1021/bi00385a022. [DOI] [PubMed] [Google Scholar]
- Schlichting I., Berendzen J., Phillips G. N., Jr, Sweet R. M. Crystal structure of photolysed carbonmonoxy-myoglobin. Nature. 1994 Oct 27;371(6500):808–812. doi: 10.1038/371808a0. [DOI] [PubMed] [Google Scholar]
- Schulte A., Bradley L., 2nd High-pressure near-infrared Raman spectroscopy of bacteriorhodopsin light to dark adaptation. Biophys J. 1995 Oct;69(4):1554–1562. doi: 10.1016/S0006-3495(95)80027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srajer V., Champion P. M. Investigations of optical line shapes and kinetic hole burning in myoglobin. Biochemistry. 1991 Jul 30;30(30):7390–7402. doi: 10.1021/bi00244a005. [DOI] [PubMed] [Google Scholar]
- Stavrov S. S. The effect of iron displacement out of the porphyrin plane on the resonance Raman spectra of heme proteins and iron porphyrins. Biophys J. 1993 Nov;65(5):1942–1950. doi: 10.1016/S0006-3495(93)81265-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach P. J., Ansari A., Berendzen J., Braunstein D., Chu K., Cowen B. R., Ehrenstein D., Frauenfelder H., Johnson J. B., Lamb D. C. Ligand binding to heme proteins: connection between dynamics and function. Biochemistry. 1991 Apr 23;30(16):3988–4001. doi: 10.1021/bi00230a026. [DOI] [PubMed] [Google Scholar]
- Steinbach P. J., Brooks B. R. Protein hydration elucidated by molecular dynamics simulation. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9135–9139. doi: 10.1073/pnas.90.19.9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timasheff S. N. The control of protein stability and association by weak interactions with water: how do solvents affect these processes? Annu Rev Biophys Biomol Struct. 1993;22:67–97. doi: 10.1146/annurev.bb.22.060193.000435. [DOI] [PubMed] [Google Scholar]
- Weber G., Drickamer H. G. The effect of high pressure upon proteins and other biomolecules. Q Rev Biophys. 1983 Feb;16(1):89–112. doi: 10.1017/s0033583500004935. [DOI] [PubMed] [Google Scholar]
- Yu N. T. Resonance Raman studies of ligand binding. Methods Enzymol. 1986;130:350–409. doi: 10.1016/0076-6879(86)30018-1. [DOI] [PubMed] [Google Scholar]
- Zipp A., Kauzmann W. Pressure denaturation of metmyoglobin. Biochemistry. 1973 Oct 9;12(21):4217–4228. doi: 10.1021/bi00745a028. [DOI] [PubMed] [Google Scholar]
- Zollfrank J., Friedrich J., Parak F. Spectral hole burning study of protoporphyrin IX substituted myoglobin. Biophys J. 1992 Mar;61(3):716–724. doi: 10.1016/S0006-3495(92)81876-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


