Abstract
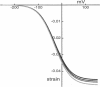
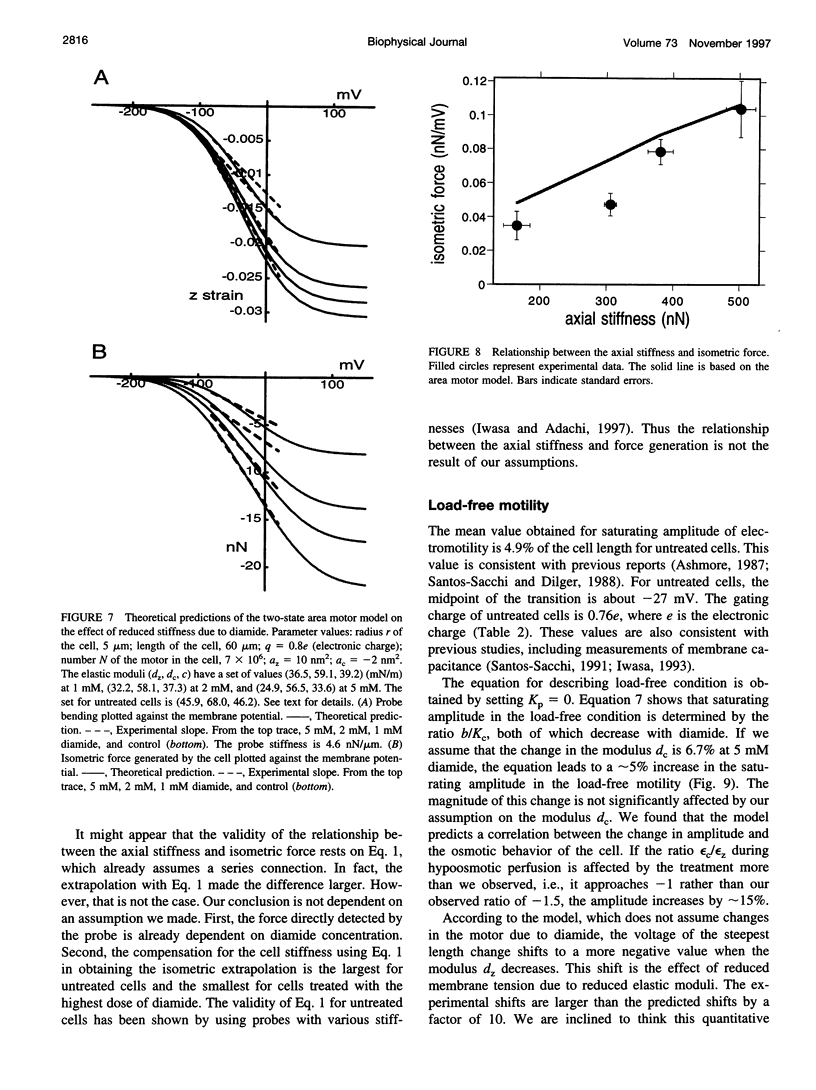
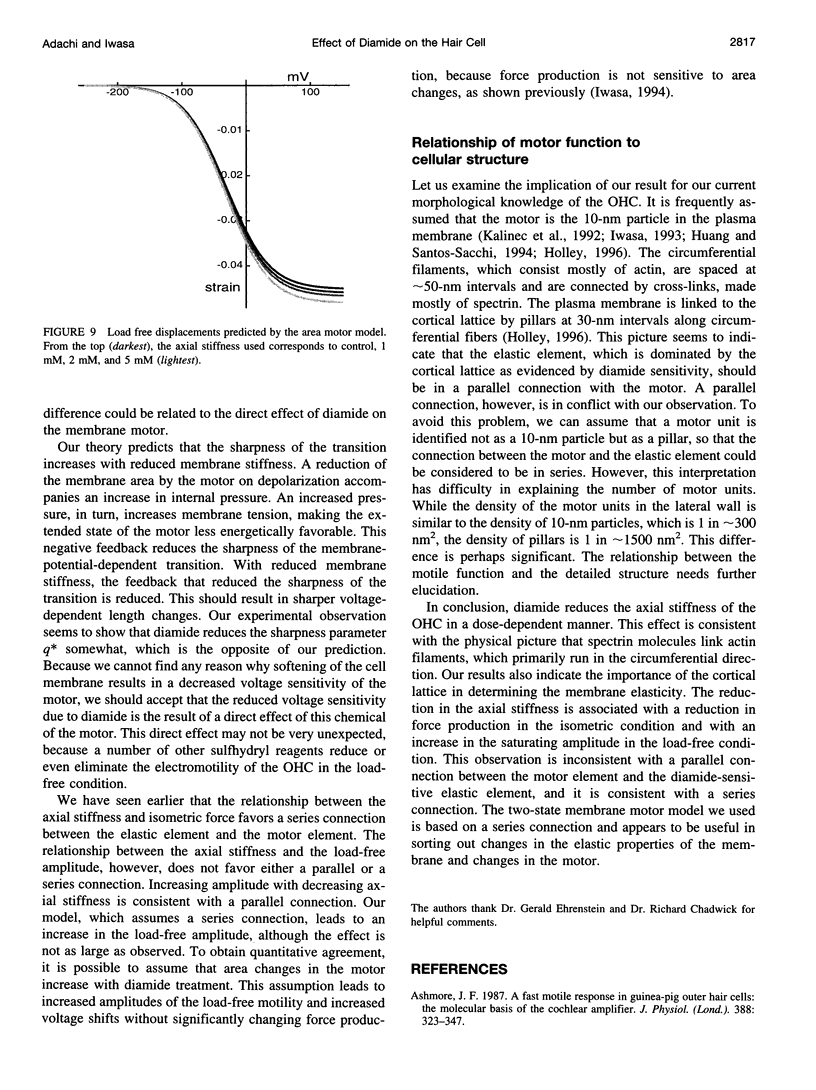
We found that diamide, which affects spectrin, reduces the axial stiffness of the cochlear outer hair cell, the cylindrically shaped mechanoreceptor cell with a unique voltage-sensitive motility. This effect thus provides a means of examining the relationship between the stiffness and the motility of the cell. For measuring axial stiffness and force production, we used an experimental configuration in which an elastic probe was attached to the cell near the cuticular plate and the other end of the cell was held with a patch pipette in the whole-cell recording mode. Diamide at concentrations of up to 5 mM reduced the axial stiffness in a dose-dependent manner to 165 nN per unit strain from 502 nN for untreated cells. The isometric force elicited by voltage pulses under whole-cell voltage clamp was also reduced to 35 pN/mV from 105 pN/mV for untreated cells. Thus the isometric force was approximately proportional to the axial stiffness. Our observations suggest a series connection between the motor and cytoskeletal elements and can be explained by the area motor model previously proposed for the outer hair cell.
Full text
PDF

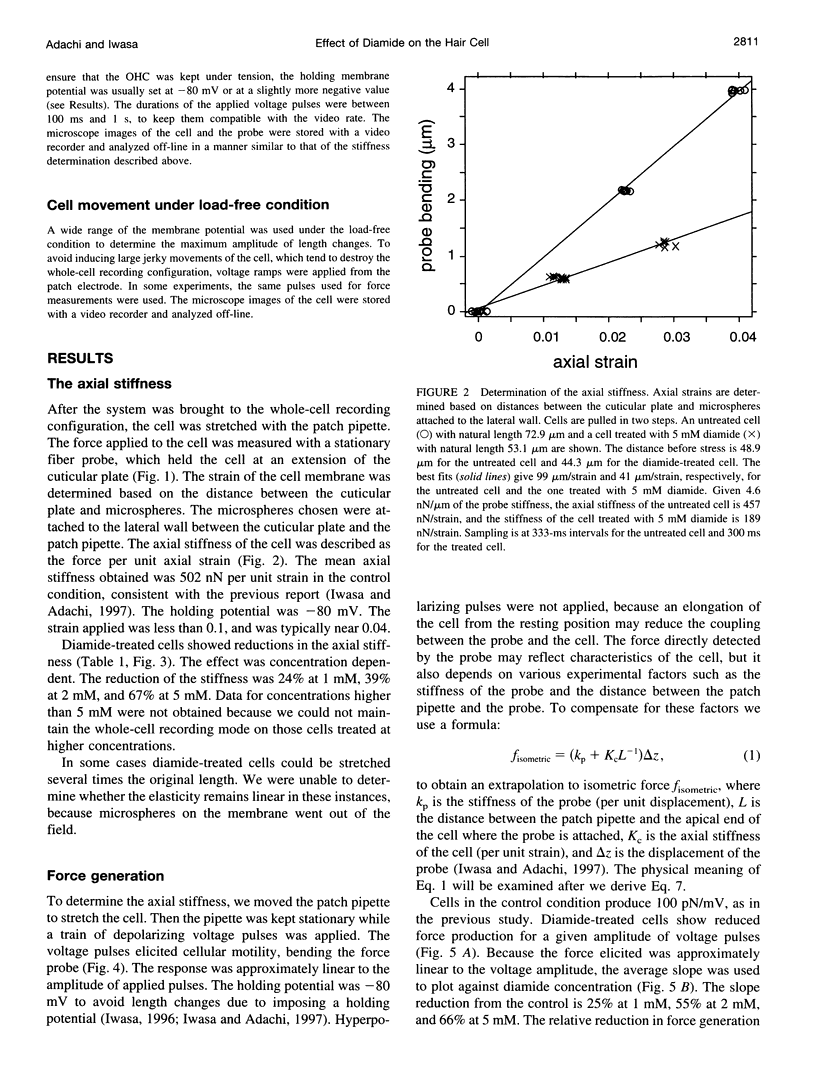



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashmore J. F. A fast motile response in guinea-pig outer hair cells: the cellular basis of the cochlear amplifier. J Physiol. 1987 Jul;388:323–347. doi: 10.1113/jphysiol.1987.sp016617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore J. F. Forward and reverse transduction in the mammalian cochlea. Neurosci Res Suppl. 1990;12:S39–S50. doi: 10.1016/0921-8696(90)90007-p. [DOI] [PubMed] [Google Scholar]
- Becker P. S., Cohen C. M., Lux S. E. The effect of mild diamide oxidation on the structure and function of human erythrocyte spectrin. J Biol Chem. 1986 Apr 5;261(10):4620–4628. [PubMed] [Google Scholar]
- Bloom M., Evans E., Mouritsen O. G. Physical properties of the fluid lipid-bilayer component of cell membranes: a perspective. Q Rev Biophys. 1991 Aug;24(3):293–397. doi: 10.1017/s0033583500003735. [DOI] [PubMed] [Google Scholar]
- Brownell W. E., Bader C. R., Bertrand D., de Ribaupierre Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science. 1985 Jan 11;227(4683):194–196. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- Dallos P., Evans B. N., Hallworth R. Nature of the motor element in electrokinetic shape changes of cochlear outer hair cells. Nature. 1991 Mar 14;350(6314):155–157. doi: 10.1038/350155a0. [DOI] [PubMed] [Google Scholar]
- Ehrenstein D., Iwasa K. H. Viscoelastic relaxation in the membrane of the auditory outer hair cell. Biophys J. 1996 Aug;71(2):1087–1094. doi: 10.1016/S0006-3495(96)79310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flock A., Flock B., Ulfendahl M. Mechanisms of movement in outer hair cells and a possible structural basis. Arch Otorhinolaryngol. 1986;243(2):83–90. doi: 10.1007/BF00453755. [DOI] [PubMed] [Google Scholar]
- Gale J. E., Ashmore J. F. Charge displacement induced by rapid stretch in the basolateral membrane of the guinea-pig outer hair cell. Proc Biol Sci. 1994 Mar 22;255(1344):243–249. doi: 10.1098/rspb.1994.0035. [DOI] [PubMed] [Google Scholar]
- Haest C. W., Kamp D., Plasa G., Deuticke B. Intra- and intermolecular cross-linking of membrane proteins in intact erythrocytes and ghosts by SH-oxidizing agents. Biochim Biophys Acta. 1977 Sep 5;469(2):226–230. doi: 10.1016/0005-2736(77)90186-9. [DOI] [PubMed] [Google Scholar]
- Hallworth R. Passive compliance and active force generation in the guinea pig outer hair cell. J Neurophysiol. 1995 Dec;74(6):2319–2328. doi: 10.1152/jn.1995.74.6.2319. [DOI] [PubMed] [Google Scholar]
- Holley M. C., Ashmore J. F. On the mechanism of a high-frequency force generator in outer hair cells isolated from the guinea pig cochlea. Proc R Soc Lond B Biol Sci. 1988 Jan 22;232(1269):413–429. doi: 10.1098/rspb.1988.0004. [DOI] [PubMed] [Google Scholar]
- Holley M. C., Ashmore J. F. Spectrin, actin and the structure of the cortical lattice in mammalian cochlear outer hair cells. J Cell Sci. 1990 Jun;96(Pt 2):283–291. doi: 10.1242/jcs.96.2.283. [DOI] [PubMed] [Google Scholar]
- Holley M. C., Kalinec F., Kachar B. Structure of the cortical cytoskeleton in mammalian outer hair cells. J Cell Sci. 1992 Jul;102(Pt 3):569–580. doi: 10.1242/jcs.102.3.569. [DOI] [PubMed] [Google Scholar]
- Huang G., Santos-Sacchi J. Motility voltage sensor of the outer hair cell resides within the lateral plasma membrane. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):12268–12272. doi: 10.1073/pnas.91.25.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa K. H. A membrane motor model for the fast motility of the outer hair cell. J Acoust Soc Am. 1994 Oct;96(4):2216–2224. doi: 10.1121/1.410094. [DOI] [PubMed] [Google Scholar]
- Iwasa K. H., Adachi M. Force generation in the outer hair cell of the cochlea. Biophys J. 1997 Jul;73(1):546–555. doi: 10.1016/S0006-3495(97)78092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa K. H., Chadwick R. S. Elasticity and active force generation of cochlear outer hair cells. J Acoust Soc Am. 1992 Dec;92(6):3169–3173. doi: 10.1121/1.404194. [DOI] [PubMed] [Google Scholar]
- Iwasa K. H. Effect of stress on the membrane capacitance of the auditory outer hair cell. Biophys J. 1993 Jul;65(1):492–498. doi: 10.1016/S0006-3495(93)81053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachar B., Brownell W. E., Altschuler R., Fex J. Electrokinetic shape changes of cochlear outer hair cells. Nature. 1986 Jul 24;322(6077):365–368. doi: 10.1038/322365a0. [DOI] [PubMed] [Google Scholar]
- Kakehata S., Santos-Sacchi J. Membrane tension directly shifts voltage dependence of outer hair cell motility and associated gating charge. Biophys J. 1995 May;68(5):2190–2197. doi: 10.1016/S0006-3495(95)80401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinec F., Holley M. C., Iwasa K. H., Lim D. J., Kachar B. A membrane-based force generation mechanism in auditory sensory cells. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8671–8675. doi: 10.1073/pnas.89.18.8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinec F., Kachar B. Inhibition of outer hair cell electromotility by sulfhydryl specific reagents. Neurosci Lett. 1993 Jul 23;157(2):231–234. doi: 10.1016/0304-3940(93)90744-6. [DOI] [PubMed] [Google Scholar]
- Knipper M., Zimmermann U., Köpschall I., Rohbock K., Jüngling S., Zenner H. P. Immunological identification of candidate proteins involved in regulating active shape changes of outer hair cells. Hear Res. 1995 Jun;86(1-2):100–110. doi: 10.1016/0378-5955(95)00060-h. [DOI] [PubMed] [Google Scholar]
- Kojima H., Ishijima A., Yanagida T. Direct measurement of stiffness of single actin filaments with and without tropomyosin by in vitro nanomanipulation. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12962–12966. doi: 10.1073/pnas.91.26.12962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M. Diamide: an oxidant probe for thiols. Methods Enzymol. 1995;251:123–133. doi: 10.1016/0076-6879(95)51116-4. [DOI] [PubMed] [Google Scholar]
- Maeda N., Kon K., Imaizumi K., Sekiya M., Shiga T. Alteration of rheological properties of human erythrocytes by crosslinking of membrane proteins. Biochim Biophys Acta. 1983 Oct 26;735(1):104–112. doi: 10.1016/0005-2736(83)90265-1. [DOI] [PubMed] [Google Scholar]
- Mammano F., Ashmore J. F. Reverse transduction measured in the isolated cochlea by laser Michelson interferometry. Nature. 1993 Oct 28;365(6449):838–841. doi: 10.1038/365838a0. [DOI] [PubMed] [Google Scholar]
- Mountain D. C. Changes in endolymphatic potential and crossed olivocochlear bundle stimulation alter cochlear mechanics. Science. 1980 Oct 3;210(4465):71–72. doi: 10.1126/science.7414321. [DOI] [PubMed] [Google Scholar]
- Nishida Y., Fujimoto T., Takagi A., Honjo I., Ogawa K. Fodrin is a constituent of the cortical lattice in outer hair cells of the guinea pig cochlea: immunocytochemical evidence. Hear Res. 1993 Feb;65(1-2):274–280. doi: 10.1016/0378-5955(93)90220-u. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J., Dilger J. P. Whole cell currents and mechanical responses of isolated outer hair cells. Hear Res. 1988 Sep 15;35(2-3):143–150. doi: 10.1016/0378-5955(88)90113-x. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J. Reversible inhibition of voltage-dependent outer hair cell motility and capacitance. J Neurosci. 1991 Oct;11(10):3096–3110. doi: 10.1523/JNEUROSCI.11-10-03096.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolomeo J. A., Steele C. R. Orthotropic piezoelectric properties of the cochlear outer hair cell wall. J Acoust Soc Am. 1995 May;97(5 Pt 1):3006–3011. doi: 10.1121/1.411865. [DOI] [PubMed] [Google Scholar]
- Xue S., Mountain D. C., Hubbard A. E. Electrically evoked basilar membrane motion. J Acoust Soc Am. 1995 May;97(5 Pt 1):3030–3041. doi: 10.1121/1.413103. [DOI] [PubMed] [Google Scholar]
- Zajic G., Schacht J. Shape changes in isolated outer hair cells: measurements with attached microspheres. Hear Res. 1991 Apr;52(2):407–410. doi: 10.1016/0378-5955(91)90029-9. [DOI] [PubMed] [Google Scholar]