Abstract
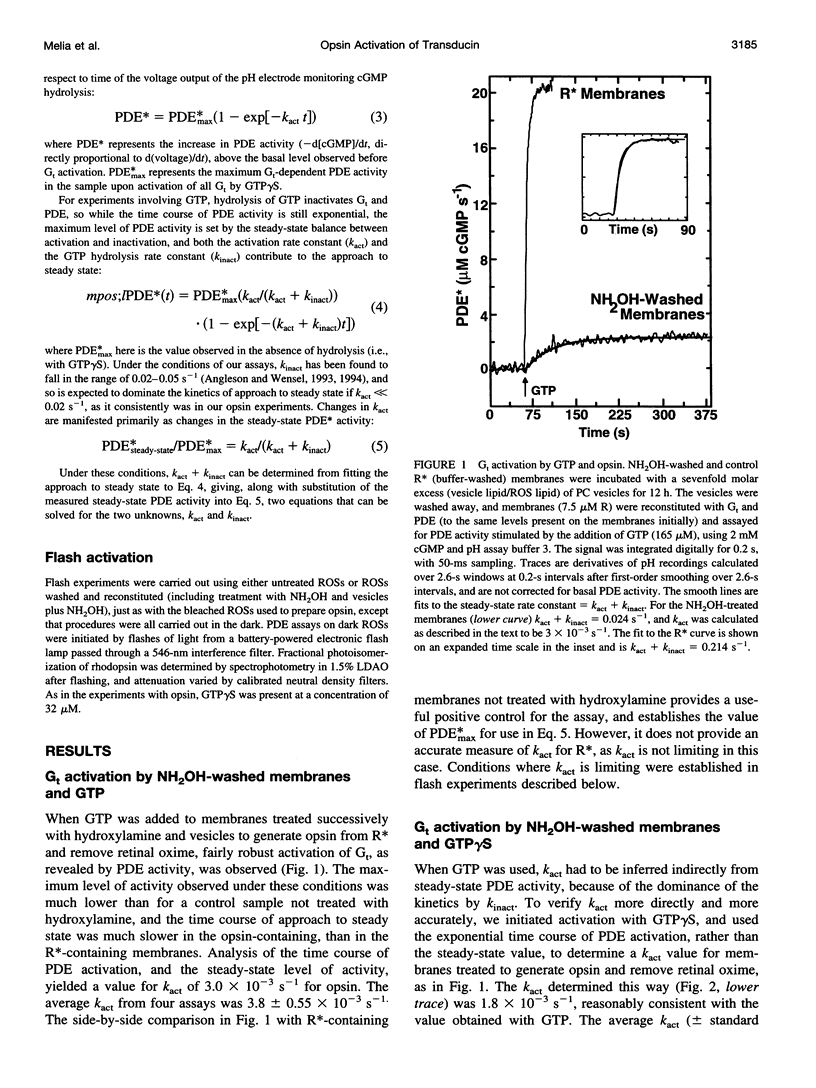
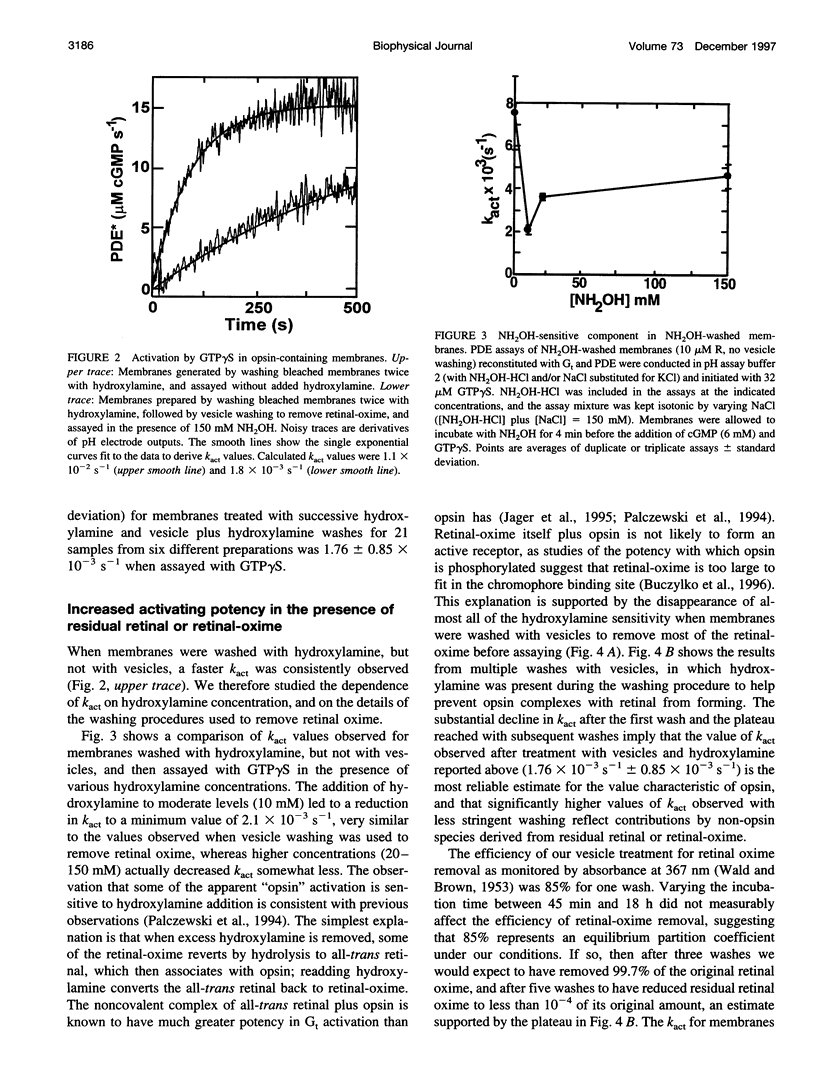
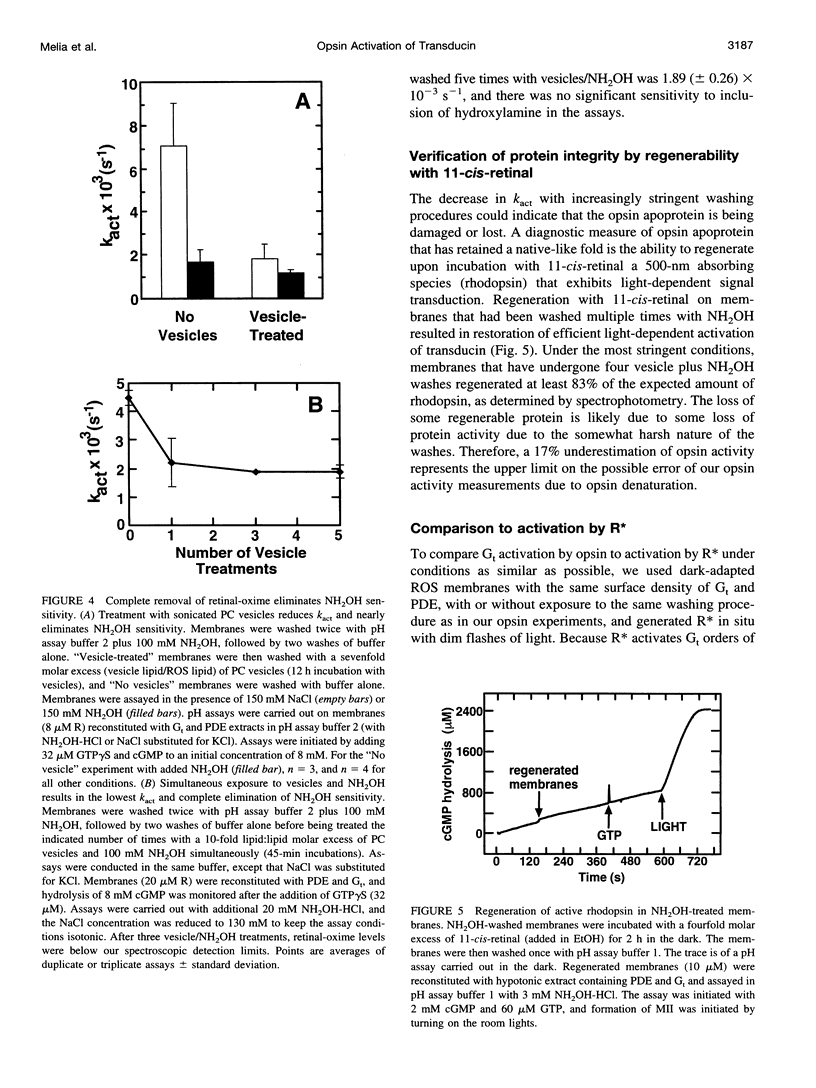
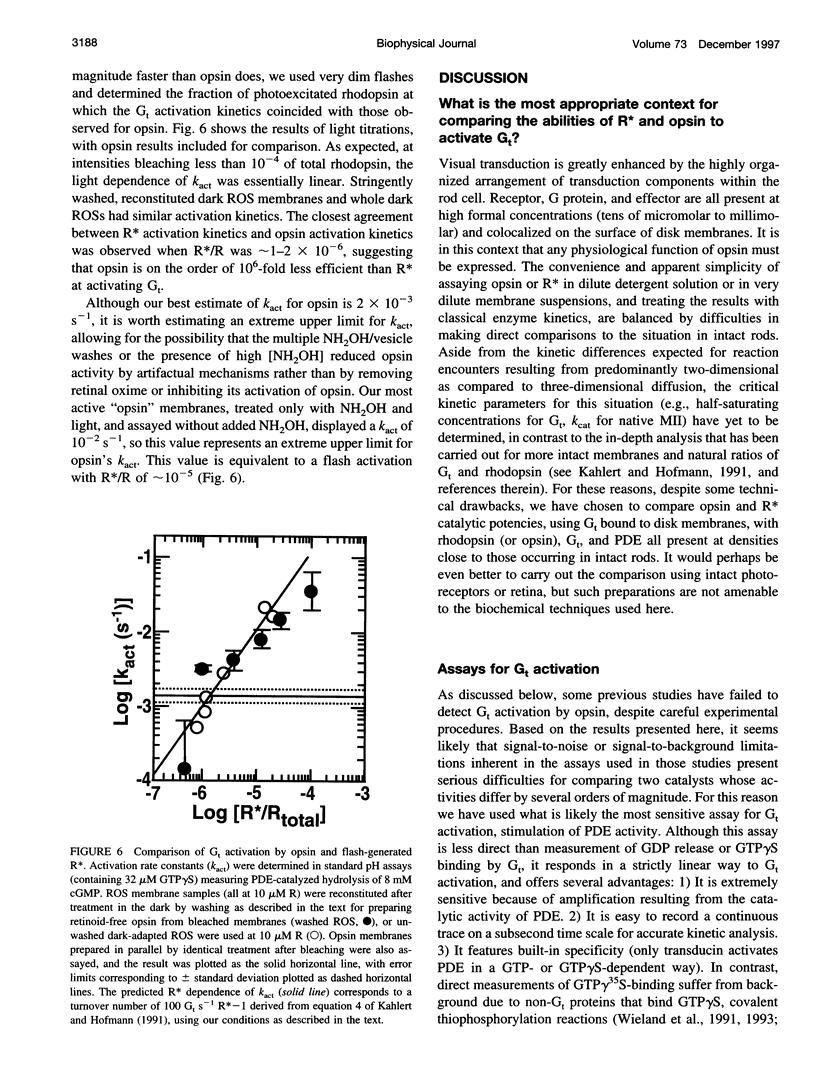
Activation of the photoreceptor G protein transducin (Gt) by opsin, the ligand-free form of rhodopsin, was measured using rod outer segment membranes with densities of opsin and Gt similar to those found in rod cells. When GTPgammaS was used as the activating nucleotide, opsin catalyzed transducin activation with an exponential time course with a rate constant k(act) on the order of 2 x 10(-3)s(-1). Comparison under these conditions to activation by flash-generated metarhodopsin II (MII) revealed that opsin- and R*-catalyzed activation showed similar kinetics when MII was present at a surface density approximately 10(-6) lower than that of opsin. Thus, in contrast to some previous reports, we find that the catalytic potency of opsin is only approximately 10(-6) that of MII. In the presence of residual retinaldehyde-derived species present in membranes treated with hydroxylamine after bleaching, the apparent k(act) observed was much higher than that for opsin, suggesting a possible explanation for previous reports of more efficient activation by opsin. These results are important for considering the possible role of opsin in the diverse phenomena in which it has been suggested to play a key role, such as bleaching desensitization and retinal degeneration induced by continuous light or vitamin A deprivation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angleson J. K., Wensel T. G. A GTPase-accelerating factor for transducin, distinct from its effector cGMP phosphodiesterase, in rod outer segment membranes. Neuron. 1993 Nov;11(5):939–949. doi: 10.1016/0896-6273(93)90123-9. [DOI] [PubMed] [Google Scholar]
- Angleson J. K., Wensel T. G. Enhancement of rod outer segment GTPase accelerating protein activity by the inhibitory subunit of cGMP phosphodiesterase. J Biol Chem. 1994 Jun 10;269(23):16290–16296. [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. Responses of retinal rods to single photons. J Physiol. 1979 Mar;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- Brin K. P., Ripps H. Rhodopsin photoproducts and rod sensitivity in the skate retina. J Gen Physiol. 1977 Jan;69(1):97–120. doi: 10.1085/jgp.69.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczyłko J., Saari J. C., Crouch R. K., Palczewski K. Mechanisms of opsin activation. J Biol Chem. 1996 Aug 23;271(34):20621–20630. doi: 10.1074/jbc.271.34.20621. [DOI] [PubMed] [Google Scholar]
- Cohen G. B., Yang T., Robinson P. R., Oprian D. D. Constitutive activation of opsin: influence of charge at position 134 and size at position 296. Biochemistry. 1993 Jun 15;32(23):6111–6115. doi: 10.1021/bi00074a024. [DOI] [PubMed] [Google Scholar]
- Cornwall M. C., Fain G. L. Bleached pigment activates transduction in isolated rods of the salamander retina. J Physiol. 1994 Oct 15;480(Pt 2):261–279. doi: 10.1113/jphysiol.1994.sp020358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corson D. W., Cornwall M. C., MacNichol E. F., Jin J., Johnson R., Derguini F., Crouch R. K., Nakanishi K. Sensitization of bleached rod photoreceptors by 11-cis-locked analogues of retinal. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6823–6827. doi: 10.1073/pnas.87.17.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOWLING J. E. Chemistry of visual adaptation in the rat. Nature. 1960 Oct 8;188:114–118. doi: 10.1038/188114a0. [DOI] [PubMed] [Google Scholar]
- Dartnall H. J. The photosensitivities of visual pigments in the presence of hydroxylamine. Vision Res. 1968 Apr;8(4):339–358. doi: 10.1016/0042-6989(68)90104-1. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Wald G. VITAMIN A DEFICIENCY AND NIGHT BLINDNESS. Proc Natl Acad Sci U S A. 1958 Jul 15;44(7):648–661. doi: 10.1073/pnas.44.7.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L., Lisman J. E. Photoreceptor degeneration in vitamin A deprivation and retinitis pigmentosa: the equivalent light hypothesis. Exp Eye Res. 1993 Sep;57(3):335–340. doi: 10.1006/exer.1993.1132. [DOI] [PubMed] [Google Scholar]
- Fawzi A. B., Northup J. K. Guanine nucleotide binding characteristics of transducin: essential role of rhodopsin for rapid exchange of guanine nucleotides. Biochemistry. 1990 Apr 17;29(15):3804–3812. doi: 10.1021/bi00467a030. [DOI] [PubMed] [Google Scholar]
- Fukada Y., Yoshizawa T. Activation of phosphodiesterase in frog rod outer segment by an intermediate of rhodopsin photolysis. II. Biochim Biophys Acta. 1981 Jul;675(2):195–200. doi: 10.1016/0304-4165(81)90226-9. [DOI] [PubMed] [Google Scholar]
- Hamm H. E., Bownds M. D. Protein complement of rod outer segments of frog retina. Biochemistry. 1986 Aug 12;25(16):4512–4523. doi: 10.1021/bi00364a010. [DOI] [PubMed] [Google Scholar]
- Hecht S., Shlaer S., Pirenne M. H. ENERGY, QUANTA, AND VISION. J Gen Physiol. 1942 Jul 20;25(6):819–840. doi: 10.1085/jgp.25.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck M., Hofmann K. P. G-protein-effector coupling: a real-time light-scattering assay for transducin-phosphodiesterase interaction. Biochemistry. 1993 Aug 17;32(32):8220–8227. doi: 10.1021/bi00083a024. [DOI] [PubMed] [Google Scholar]
- Hofmann K. P., Emeis D., Schnetkamp P. P. Interplay between hydroxylamine, metarhodopsin II and GTP-binding protein in bovine photoreceptor membranes. Biochim Biophys Acta. 1983 Oct 31;725(1):60–70. doi: 10.1016/0005-2728(83)90224-4. [DOI] [PubMed] [Google Scholar]
- Hofmann K. P., Pulvermüller A., Buczyłko J., Van Hooser P., Palczewski K. The role of arrestin and retinoids in the regeneration pathway of rhodopsin. J Biol Chem. 1992 Aug 5;267(22):15701–15706. [PubMed] [Google Scholar]
- Jin J., Crouch R. K., Corson D. W., Katz B. M., MacNichol E. F., Cornwall M. C. Noncovalent occupancy of the retinal-binding pocket of opsin diminishes bleaching adaptation of retinal cones. Neuron. 1993 Sep;11(3):513–522. doi: 10.1016/0896-6273(93)90155-k. [DOI] [PubMed] [Google Scholar]
- Jäger S., Palczewski K., Hofmann K. P. Opsin/all-trans-retinal complex activates transducin by different mechanisms than photolyzed rhodopsin. Biochemistry. 1996 Mar 5;35(9):2901–2908. doi: 10.1021/bi9524068. [DOI] [PubMed] [Google Scholar]
- Kahlert M., Hofmann K. P. Reaction rate and collisional efficiency of the rhodopsin-transducin system in intact retinal rods. Biophys J. 1991 Feb;59(2):375–386. doi: 10.1016/S0006-3495(91)82231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnik S. S., Ridge K. D., Bhattacharya S., Khorana H. G. Palmitoylation of bovine opsin and its cysteine mutants in COS cells. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):40–44. doi: 10.1073/pnas.90.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen T. J., Inglehearn C. F., Lester D. H., Bashir R., Jay M., Bird A. C., Jay B., Bhattacharya S. S. Autosomal dominant retinitis pigmentosa: four new mutations in rhodopsin, one of them in the retinal attachment site. Genomics. 1991 Sep;11(1):199–205. doi: 10.1016/0888-7543(91)90119-y. [DOI] [PubMed] [Google Scholar]
- Lamb T. D., Pugh E. N., Jr A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J Physiol. 1992 Apr;449:719–758. doi: 10.1113/jphysiol.1992.sp019111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman P. A., Evanczuk A. T. Real time assay of rod disk membrane cGMP phosphodiesterase and its controller enzymes. Methods Enzymol. 1982;81:532–542. doi: 10.1016/s0076-6879(82)81074-4. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Parker K. R., Dratz E. A. The molecular mechanism of visual excitation and its relation to the structure and composition of the rod outer segment. Annu Rev Physiol. 1987;49:765–791. doi: 10.1146/annurev.ph.49.030187.004001. [DOI] [PubMed] [Google Scholar]
- Malinski J. A., Wensel T. G. Membrane stimulation of cGMP phosphodiesterase activation by transducin: comparison of phospholipid bilayers to rod outer segment membranes. Biochemistry. 1992 Oct 6;31(39):9502–9512. doi: 10.1021/bi00154a024. [DOI] [PubMed] [Google Scholar]
- Malinski J. A., Zera E. M., Angleson J. K., Wensel T. G. High affinity interactions of GTPgammaS with the heterotrimeric G protein, transducin. Evidence at high and low protein concentrations. J Biol Chem. 1996 May 31;271(22):12919–12924. doi: 10.1074/jbc.271.22.12919. [DOI] [PubMed] [Google Scholar]
- Matthews H. R., Cornwall M. C., Fain G. L. Persistent activation of transducin by bleached rhodopsin in salamander rods. J Gen Physiol. 1996 Dec;108(6):557–563. doi: 10.1085/jgp.108.6.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milano C. A., Allen L. F., Rockman H. A., Dolber P. C., McMinn T. R., Chien K. R., Johnson T. D., Bond R. A., Lefkowitz R. J. Enhanced myocardial function in transgenic mice overexpressing the beta 2-adrenergic receptor. Science. 1994 Apr 22;264(5158):582–586. doi: 10.1126/science.8160017. [DOI] [PubMed] [Google Scholar]
- Morrison D. F., O'Brien P. J., Pepperberg D. R. Depalmitylation with hydroxylamine alters the functional properties of rhodopsin. J Biol Chem. 1991 Oct 25;266(30):20118–20123. [PubMed] [Google Scholar]
- Nakayama T. A., Khorana H. G. Mapping of the amino acids in membrane-embedded helices that interact with the retinal chromophore in bovine rhodopsin. J Biol Chem. 1991 Mar 5;266(7):4269–4275. [PubMed] [Google Scholar]
- Noell W. K., Delmelle M. C., Albrecht R. Vitamin A deficiency effect on retina: dependence on light. Science. 1971 Apr 2;172(3978):72–75. doi: 10.1126/science.172.3978.72. [DOI] [PubMed] [Google Scholar]
- Okada D., Nakai T., Ikai A. Transducin activation by molecular species of rhodopsin other than metarhodopsin II. Photochem Photobiol. 1989 Feb;49(2):197–203. doi: 10.1111/j.1751-1097.1989.tb04096.x. [DOI] [PubMed] [Google Scholar]
- Palczewski K., Jäger S., Buczyłko J., Crouch R. K., Bredberg D. L., Hofmann K. P., Asson-Batres M. A., Saari J. C. Rod outer segment retinol dehydrogenase: substrate specificity and role in phototransduction. Biochemistry. 1994 Nov 22;33(46):13741–13750. doi: 10.1021/bi00250a027. [DOI] [PubMed] [Google Scholar]
- Papermaster D. S., Dreyer W. J. Rhodopsin content in the outer segment membranes of bovine and frog retinal rods. Biochemistry. 1974 May 21;13(11):2438–2444. doi: 10.1021/bi00708a031. [DOI] [PubMed] [Google Scholar]
- Pepperberg D. R., Brown P. K., Lurie M., Dowling J. E. Visual pigment and photoreceptor sensitivity in the isolated skate retina. J Gen Physiol. 1978 Apr;71(4):369–396. doi: 10.1085/jgp.71.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh E. N., Jr, Lamb T. D. Amplification and kinetics of the activation steps in phototransduction. Biochim Biophys Acta. 1993 Mar 1;1141(2-3):111–149. doi: 10.1016/0005-2728(93)90038-h. [DOI] [PubMed] [Google Scholar]
- RUSHTON W. A. Rhodopsin measurement and dark-adaptation in a subject deficient in cone vision. J Physiol. 1961 Apr;156:193–205. doi: 10.1113/jphysiol.1961.sp006668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramdas L., Disher R. M., Wensel T. G. Nucleotide exchange and cGMP phosphodiesterase activation by pertussis toxin inactivated transducin. Biochemistry. 1991 Dec 17;30(50):11637–11645. doi: 10.1021/bi00114a005. [DOI] [PubMed] [Google Scholar]
- Ratner V. L., Bagirov I. G., Fesenko E. E. Metarhodopsin I can react with hydroxylamine. Vision Res. 1981;21(2):251–253. doi: 10.1016/0042-6989(81)90118-8. [DOI] [PubMed] [Google Scholar]
- Samama P., Cotecchia S., Costa T., Lefkowitz R. J. A mutation-induced activated state of the beta 2-adrenergic receptor. Extending the ternary complex model. J Biol Chem. 1993 Mar 5;268(7):4625–4636. [PubMed] [Google Scholar]
- Stubbs G. W., Litman B. J. Microviscosity of the hydrocarbon region of the bovine retinal rod outer segment disk membrane determined by fluorescent probe measurements. Biochemistry. 1976 Jun 29;15(13):2766–2772. doi: 10.1021/bi00658a009. [DOI] [PubMed] [Google Scholar]
- Surya A., Foster K. W., Knox B. E. Transducin activation by the bovine opsin apoprotein. J Biol Chem. 1995 Mar 10;270(10):5024–5031. doi: 10.1074/jbc.270.10.5024. [DOI] [PubMed] [Google Scholar]
- Tyminski P. N., O'Brien D. F. Rod outer segment phosphodiesterase binding and activation in reconstituted membranes. Biochemistry. 1984 Aug 14;23(17):3986–3993. doi: 10.1021/bi00312a028. [DOI] [PubMed] [Google Scholar]
- Vuong T. M., Chabre M., Stryer L. Millisecond activation of transducin in the cyclic nucleotide cascade of vision. Nature. 1984 Oct 18;311(5987):659–661. doi: 10.1038/311659a0. [DOI] [PubMed] [Google Scholar]
- WALD G., BROWN P. K. The molar extinction of rhodopsin. J Gen Physiol. 1953 Nov 20;37(2):189–200. doi: 10.1085/jgp.37.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessling-Resnick M., Johnson G. L. Transducin interactions with rhodopsin. Evidence for positive cooperative behavior. J Biol Chem. 1987 Sep 15;262(26):12444–12447. [PubMed] [Google Scholar]
- Wieland T., Nürnberg B., Ulibarri I., Kaldenberg-Stasch S., Schultz G., Jakobs K. H. Guanine nucleotide-specific phosphate transfer by guanine nucleotide-binding regulatory protein beta-subunits. Characterization of the phosphorylated amino acid. J Biol Chem. 1993 Aug 25;268(24):18111–18118. [PubMed] [Google Scholar]
- Wieland T., Ulibarri I., Gierschik P., Jakobs K. H. Activation of signal-transducing guanine-nucleotide-binding regulatory proteins by guanosine 5'-[gamma-thio]triphosphate. Information transfer by intermediately thiophosphorylated beta gamma subunits. Eur J Biochem. 1991 Mar 28;196(3):707–716. doi: 10.1111/j.1432-1033.1991.tb15869.x. [DOI] [PubMed] [Google Scholar]
- Zhukovsky E. A., Robinson P. R., Oprian D. D. Transducin activation by rhodopsin without a covalent bond to the 11-cis-retinal chromophore. Science. 1991 Feb 1;251(4993):558–560. doi: 10.1126/science.1990431. [DOI] [PubMed] [Google Scholar]


