Abstract
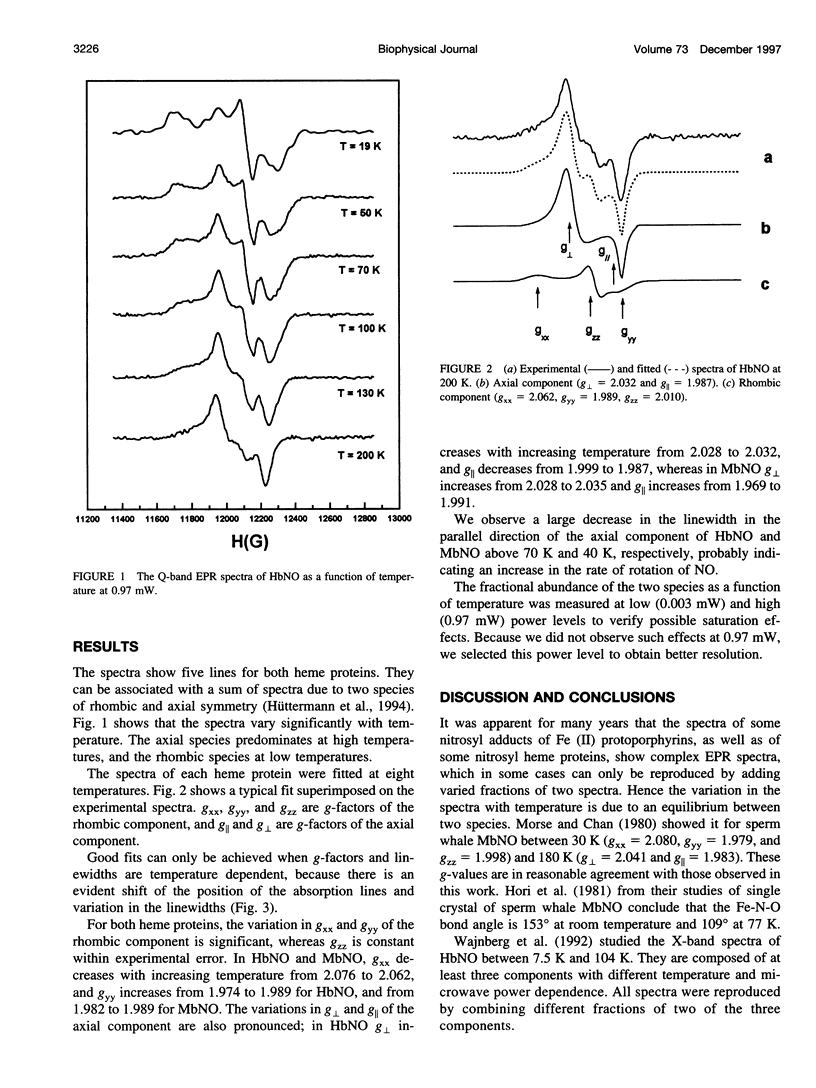
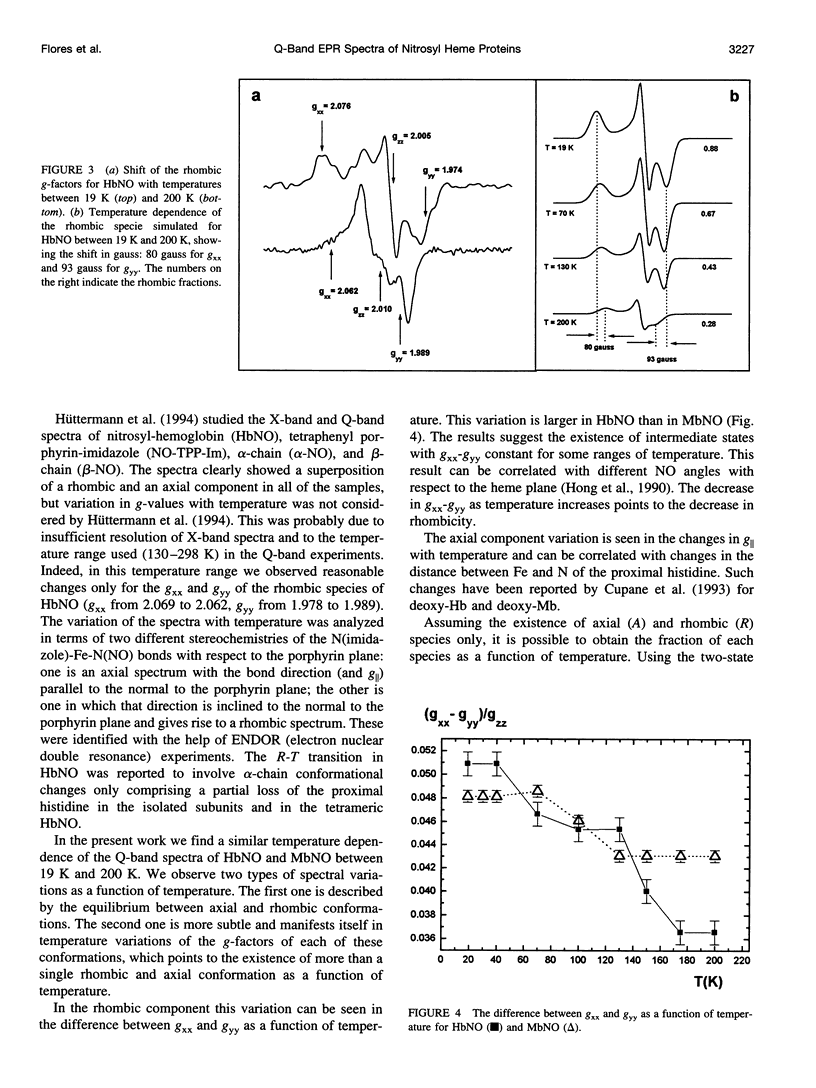
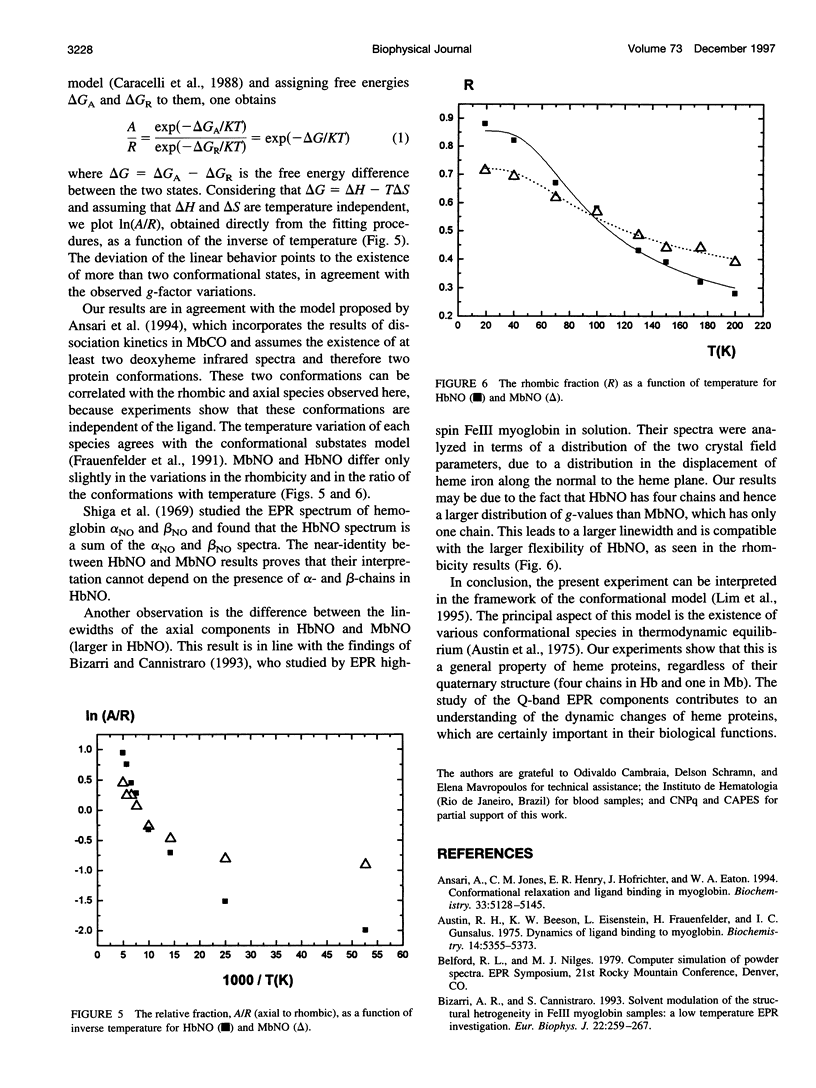
The Q-band (35 GHz) electron paramagnetic resonance (EPR) spectra of nitrosyl hemoglobin (HbNO) and nitrosyl myoglobin (MbNO) were studied as a function of temperature between 19 K and 200 K. The spectra of both heme proteins show two classes of variations as a function of temperature. The first one has previously been associated with the existence of two paramagnetic species, one with rhombic and the other with axial symmetry. The second one manifests itself in changes in the g-factors and linewidths of each species. These changes are correlated with the conformational substates model and associate the variations of g-values with changes in the angle of the N(his)-Fe-N(NO) bond in the rhombic species and with changes in the distance between Fe and N of the proximal (F8) histidine in the axial species.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansari A., Jones C. M., Henry E. R., Hofrichter J., Eaton W. A. Conformational relaxation and ligand binding in myoglobin. Biochemistry. 1994 May 3;33(17):5128–5145. doi: 10.1021/bi00183a017. [DOI] [PubMed] [Google Scholar]
- Austin R. H., Beeson K. W., Eisenstein L., Frauenfelder H., Gunsalus I. C. Dynamics of ligand binding to myoglobin. Biochemistry. 1975 Dec 2;14(24):5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- Bizzarri A. R., Cannistraro S. Solvent modulation of the structural heterogeneity in FeIII myoglobin samples: a low temperature EPR investigation. Eur Biophys J. 1993;22(4):259–267. doi: 10.1007/BF00180260. [DOI] [PubMed] [Google Scholar]
- Caracelli I., Meirelles N. C., Tabak M., Baffa Filho O., Nascimento O. R. An ESR study of nitrosyl-Aplysia brasiliana myoglobin and nitrosyl annelidae Glossoscolex paulistus erythrocruorin. Biochim Biophys Acta. 1988 Aug 10;955(3):315–320. doi: 10.1016/0167-4838(88)90210-5. [DOI] [PubMed] [Google Scholar]
- Chevion M., Stern A., Peisach J., Blumberg W. E., Simon S. Analogous effect of protons and inositol hexaphosphate on the alteration of structure of nitrosyl fetal human hemoglobin. Biochemistry. 1978 May 2;17(9):1745–1750. doi: 10.1021/bi00602a025. [DOI] [PubMed] [Google Scholar]
- Cupane A., Leone M., Vitrano E. Protein dynamics: conformational disorder, vibrational coupling and anharmonicity in deoxy-hemoglobin and myoglobin. Eur Biophys J. 1993;21(6):385–391. doi: 10.1007/BF00185865. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Sligar S. G., Wolynes P. G. The energy landscapes and motions of proteins. Science. 1991 Dec 13;254(5038):1598–1603. doi: 10.1126/science.1749933. [DOI] [PubMed] [Google Scholar]
- Henry Y., Banerjee R. Electron paramagnetic studies of nitric oxide haemoglobin derivatives: isolated subunits and nitric oxide hybrids. J Mol Biol. 1973 Feb 5;73(4):469–482. doi: 10.1016/0022-2836(73)90094-6. [DOI] [PubMed] [Google Scholar]
- Honeycutt J. D., Thirumalai D. Metastability of the folded states of globular proteins. Proc Natl Acad Sci U S A. 1990 May;87(9):3526–3529. doi: 10.1073/pnas.87.9.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M. K., Braunstein D., Cowen B. R., Frauenfelder H., Iben I. E., Mourant J. R., Ormos P., Scholl R., Schulte A., Steinbach P. J. Conformational substates and motions in myoglobin. External influences on structure and dynamics. Biophys J. 1990 Aug;58(2):429–436. doi: 10.1016/S0006-3495(90)82388-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori H., Ikeda-Saito M., Yonetani T. Single crystal EPR of myoglobin nitroxide. Freezing-induced reversible changes in the molecular orientation of the ligand. J Biol Chem. 1981 Aug 10;256(15):7849–7855. [PubMed] [Google Scholar]
- Jia L., Bonaventura C., Bonaventura J., Stamler J. S. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature. 1996 Mar 21;380(6571):221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- Lim M., Jackson T. A., Anfinrud P. A. Binding of CO to myoglobin from a heme pocket docking site to form nearly linear Fe-C-O. Science. 1995 Aug 18;269(5226):962–966. doi: 10.1126/science.7638619. [DOI] [PubMed] [Google Scholar]
- Louro S. R., Ribeiro P. C., Bemski G. EPR spectral changes of nitrosyl hemes and their relation to the hemoglobin T-R transition. Biochim Biophys Acta. 1981 Aug 28;670(1):56–63. doi: 10.1016/0005-2795(81)90048-9. [DOI] [PubMed] [Google Scholar]
- Morse R. H., Chan S. I. Electron paramagnetic resonance studies of nitrosyl ferrous heme complexes. Determination of an equilibrium between two conformations. J Biol Chem. 1980 Aug 25;255(16):7876–7882. [PubMed] [Google Scholar]
- Mun S. K., Chang J. C., Das T. P. Origin of observed changes in 14N hyperfine interaction accompanying R leads to T transition in nitrosylhemoglobin. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4842–4846. doi: 10.1073/pnas.76.10.4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto L. M., Nascimento O. R., Tabak M., Caracelli I. The mechanism of reaction of nitrosyl with met- and oxymyoglobin: an ESR study. Biochim Biophys Acta. 1988 Sep 21;956(2):189–196. doi: 10.1016/0167-4838(88)90265-8. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Kilmartin J. V., Nagai K., Szabo A., Simon S. R. Influence of globin structures on the state of the heme. Ferrous low spin derivatives. Biochemistry. 1976 Jan 27;15(2):378–387. doi: 10.1021/bi00647a022. [DOI] [PubMed] [Google Scholar]
- Potter W. T., Hazzard J. H., Choc M. G., Tucker M. P., Caughey W. S. Infrared spectra of carbonyl hemoglobins: characterization of dynamic heme pocket conformers. Biochemistry. 1990 Jul 3;29(26):6283–6295. doi: 10.1021/bi00478a025. [DOI] [PubMed] [Google Scholar]
- Shiga T., Hwang K. J., Tyuma I. Electron paramagnetic resonance studies of nitric oxide hemoglobin derivatives. I. Human hemoglobin subunits. Biochemistry. 1969 Jan;8(1):378–383. doi: 10.1021/bi00829a052. [DOI] [PubMed] [Google Scholar]
- Wajnberg E., Bemski G., el-Jaick L. J., Alves O. C. Nitrosyl hemoglobins: EPR above 80 K. Int J Biol Macromol. 1996 Apr;18(3):231–235. doi: 10.1016/0141-8130(95)01078-5. [DOI] [PubMed] [Google Scholar]
- Wajnberg E., Linhares M. P., el-Jaick L. J., Bemski G. Nitrosyl hemoglobin: EPR components at low temperatures. Eur Biophys J. 1992;21(1):57–61. doi: 10.1007/BF00195444. [DOI] [PubMed] [Google Scholar]


