Abstract
In a complex of two electron-transfer proteins, their redox potentials can be shifted due to changes in the dielectric surroundings and the electrostatic potentials at each center caused by the charged residues of the partner. These effects are dependent on the geometry of the complex. Three different docking configurations (DCs) for intracomplex electron transfer between cytochrome f and plastocyanin were studied, defined by 1) close contact of the positively charged region of cytochrome f and the negatively charged regions of plastocyanin (DC1) and by (2, 3) close contact of the surface regions adjacent to the Fe and Cu redox centers (DC2 and DC3). The equilibrium energetics for electron transfer in DC1-DC3 are the same within approximately +/-0.1 kT. The lower reorganization energy for DC2 results in a slightly lower activation energy for this complex compared with DC1 and DC3. The long heme-copper distance (approximately 24 A) in the DC1 complex drastically decreases electronic coupling and makes this complex much less favorable for electron transfer than DC2 or DC3. DC1-like complexes can only serve as docking intermediates in the pathway toward formation of an electron-transfer-competent complex. Elimination of the four positive charges arising from the lysine residues in the positive patch of cytochrome f, as accomplished by mutagenesis, exerts a negligible effect (approximately 3 mV) on the redox potential difference between cyt f and PC.
Full text
PDF


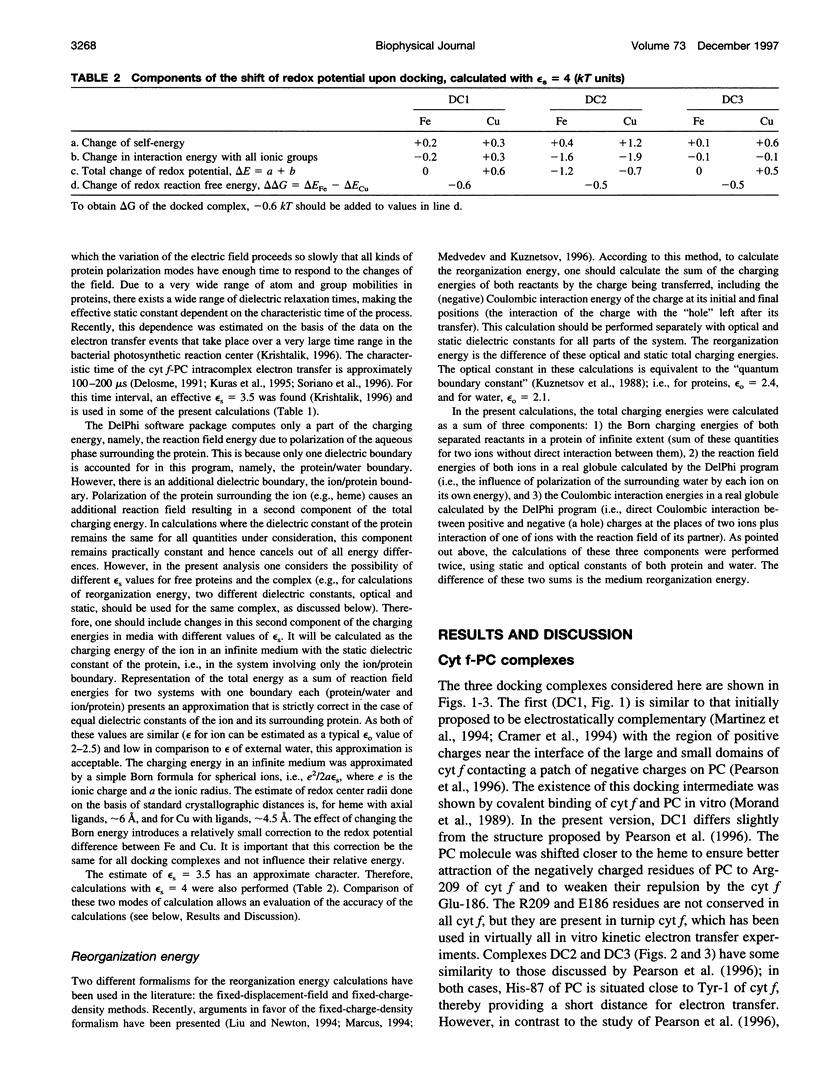
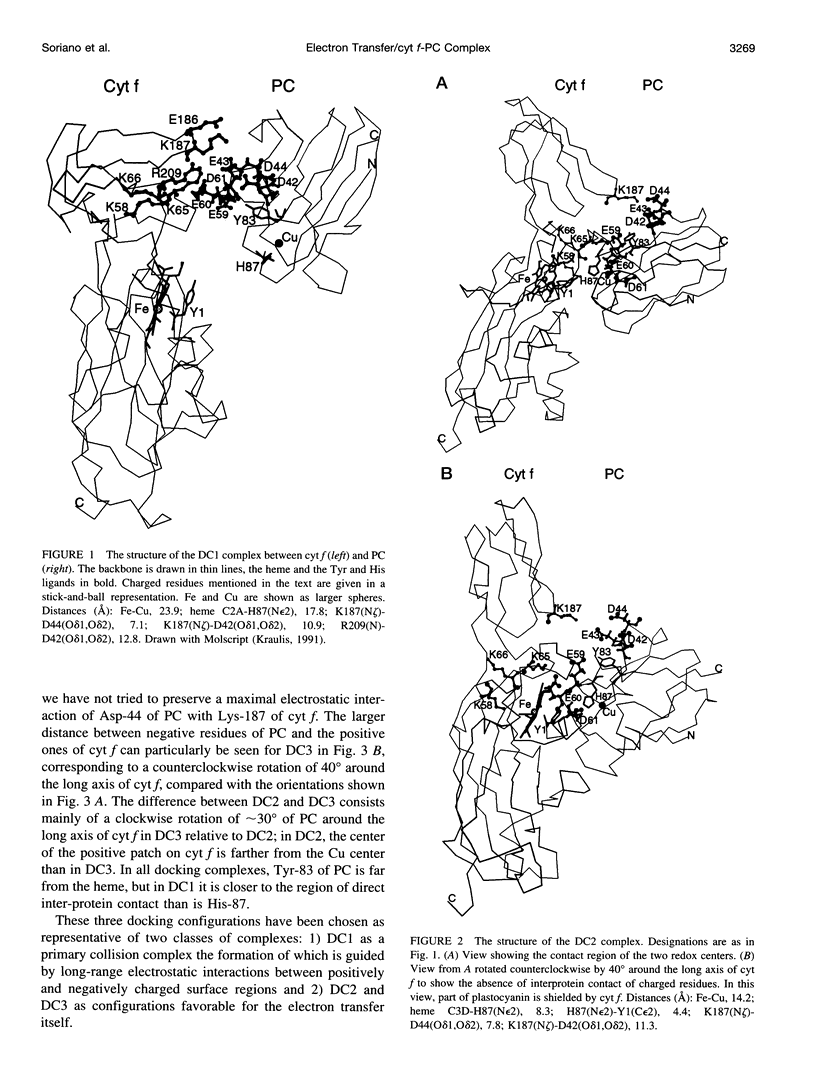
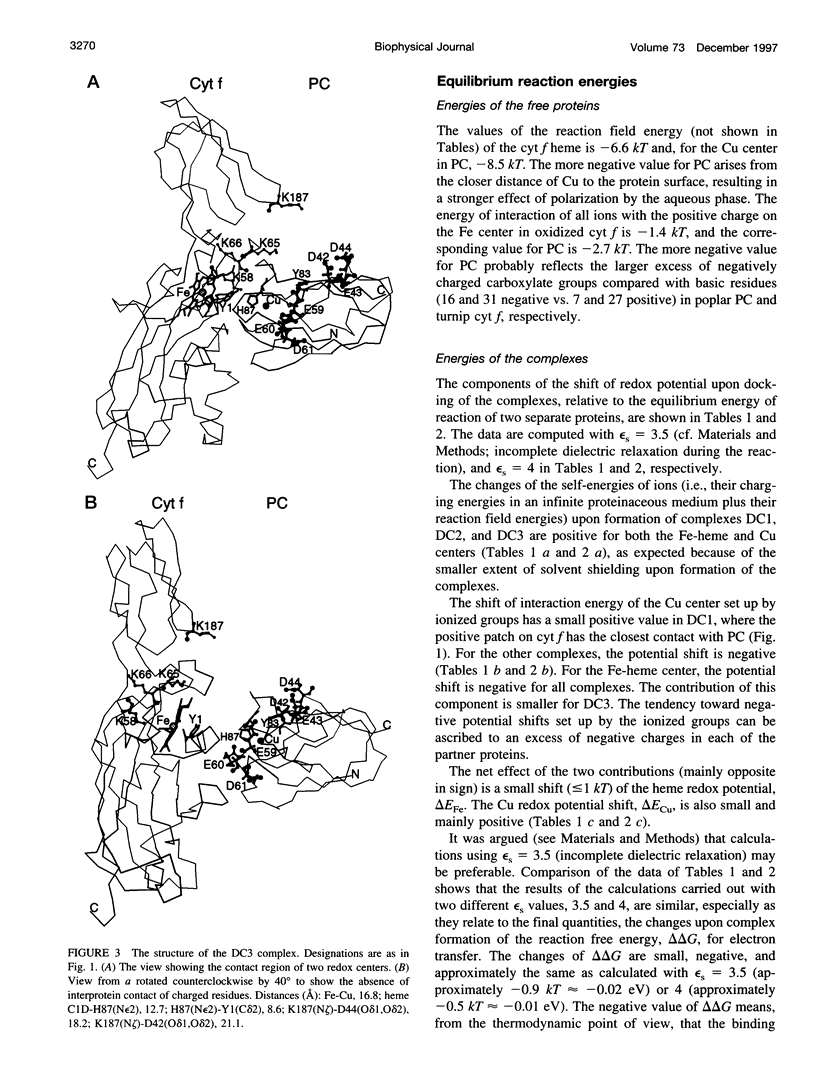






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagby S., Barker P. D., Guo L. H., Hill H. A. Direct electrochemistry of protein-protein complexes involving cytochrome c, cytochrome b5, and plastocyanin. Biochemistry. 1990 Apr 3;29(13):3213–3219. doi: 10.1021/bi00465a010. [DOI] [PubMed] [Google Scholar]
- Bagby S., Driscoll P. C., Goodall K. G., Redfield C., Hill H. A. The complex formed between plastocyanin and cytochrome c. Investigation by NMR spectroscopy. Eur J Biochem. 1990 Mar 10;188(2):413–420. doi: 10.1111/j.1432-1033.1990.tb15418.x. [DOI] [PubMed] [Google Scholar]
- Bagby S., Driscoll P. C., Harvey T. S., Hill H. A. High-resolution solution structure of reduced parsley plastocyanin. Biochemistry. 1994 May 31;33(21):6611–6622. doi: 10.1021/bi00187a031. [DOI] [PubMed] [Google Scholar]
- Burrows A. L., Guo L. H., Hill H. A., McLendon G., Sherman F. Direct electrochemistry of proteins. Investigations of yeast cytochrome c mutants and their complexes with cytochrome b5. Eur J Biochem. 1991 Dec 5;202(2):543–549. doi: 10.1111/j.1432-1033.1991.tb16406.x. [DOI] [PubMed] [Google Scholar]
- Cramer W. A., Soriano G. M., Ponomarev M., Huang D., Zhang H., Martinez S. E., Smith J. L. SOME NEW STRUCTURAL ASPECTS AND OLD CONTROVERSIES CONCERNING THE CYTOCHROME b6f COMPLEX OF OXYGENIC PHOTOSYNTHESIS. Annu Rev Plant Physiol Plant Mol Biol. 1996 Jun;47(NaN):477–508. doi: 10.1146/annurev.arplant.47.1.477. [DOI] [PubMed] [Google Scholar]
- DAVENPORT H. E., HILL R. The preparation and some properties of cytochrome f. Proc R Soc Lond B Biol Sci. 1952 Apr 24;139(896):327–345. doi: 10.1098/rspb.1952.0016. [DOI] [PubMed] [Google Scholar]
- Drepper F., Hippler M., Nitschke W., Haehnel W. Binding dynamics and electron transfer between plastocyanin and photosystem I. Biochemistry. 1996 Jan 30;35(4):1282–1295. doi: 10.1021/bi951471e. [DOI] [PubMed] [Google Scholar]
- Farah J., Rappaport F., Choquet Y., Joliot P., Rochaix J. D. Isolation of a psaF-deficient mutant of Chlamydomonas reinhardtii: efficient interaction of plastocyanin with the photosystem I reaction center is mediated by the PsaF subunit. EMBO J. 1995 Oct 16;14(20):4976–4984. doi: 10.1002/j.1460-2075.1995.tb00180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray H. B., Winkler J. R. Electron transfer in proteins. Annu Rev Biochem. 1996;65:537–561. doi: 10.1146/annurev.bi.65.070196.002541. [DOI] [PubMed] [Google Scholar]
- Guss J. M., Bartunik H. D., Freeman H. C. Accuracy and precision in protein structure analysis: restrained least-squares refinement of the structure of poplar plastocyanin at 1.33 A resolution. Acta Crystallogr B. 1992 Dec 1;48(Pt 6):790–811. doi: 10.1107/s0108768192004270. [DOI] [PubMed] [Google Scholar]
- Guss J. M., Freeman H. C. Structure of oxidized poplar plastocyanin at 1.6 A resolution. J Mol Biol. 1983 Sep 15;169(2):521–563. doi: 10.1016/s0022-2836(83)80064-3. [DOI] [PubMed] [Google Scholar]
- Haehnel W., Jansen T., Gause K., Klösgen R. B., Stahl B., Michl D., Huvermann B., Karas M., Herrmann R. G. Electron transfer from plastocyanin to photosystem I. EMBO J. 1994 Mar 1;13(5):1028–1038. doi: 10.1002/j.1460-2075.1994.tb06351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S., Modi S., Bendall D. S., Gray J. C. The surface-exposed tyrosine residue Tyr83 of pea plastocyanin is involved in both binding and electron transfer reactions with cytochrome f. EMBO J. 1991 Dec;10(13):4011–4016. doi: 10.1002/j.1460-2075.1991.tb04976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtalik L. I., Kuznetsov A. M., Mertz E. L. Electrostatics of proteins: description in terms of two dielectric constants simultaneously. Proteins. 1997 Jun;28(2):174–182. [PubMed] [Google Scholar]
- Kuras R., Wollman F. A., Joliot P. Conversion of cytochrome f to a soluble form in vivo in Chlamydomonas reinhardtii. Biochemistry. 1995 Jun 6;34(22):7468–7475. doi: 10.1021/bi00022a021. [DOI] [PubMed] [Google Scholar]
- Leonard J. J., Yonetani T. Interaction of cytochrome c peroxidase with cytochrome c. Biochemistry. 1974 Mar 26;13(7):1465–1468. doi: 10.1021/bi00704a023. [DOI] [PubMed] [Google Scholar]
- Martinez S. E., Huang D., Ponomarev M., Cramer W. A., Smith J. L. The heme redox center of chloroplast cytochrome f is linked to a buried five-water chain. Protein Sci. 1996 Jun;5(6):1081–1092. doi: 10.1002/pro.5560050610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez S. E., Huang D., Szczepaniak A., Cramer W. A., Smith J. L. Crystal structure of chloroplast cytochrome f reveals a novel cytochrome fold and unexpected heme ligation. Structure. 1994 Feb 15;2(2):95–105. doi: 10.1016/s0969-2126(00)00012-5. [DOI] [PubMed] [Google Scholar]
- Metzger S. U., Cramer W. A., Whitmarsh J. Critical analysis of the extinction coefficient of chloroplast cytochrome f. Biochim Biophys Acta. 1997 Apr 11;1319(2-3):233–241. doi: 10.1016/s0005-2728(96)00164-8. [DOI] [PubMed] [Google Scholar]
- Meyer T. E., Zhao Z. G., Cusanovich M. A., Tollin G. Transient kinetics of electron transfer from a variety of c-type cytochromes to plastocyanin. Biochemistry. 1993 May 4;32(17):4552–4559. doi: 10.1021/bi00068a010. [DOI] [PubMed] [Google Scholar]
- Modi S., Nordling M., Lundberg L. G., Hansson O., Bendall D. S. Reactivity of cytochromes c and f with mutant forms of spinach plastocyanin. Biochim Biophys Acta. 1992 Aug 28;1102(1):85–90. doi: 10.1016/0005-2728(92)90068-d. [DOI] [PubMed] [Google Scholar]
- Morand L. Z., Frame M. K., Colvert K. K., Johnson D. A., Krogmann D. W., Davis D. J. Plastocyanin cytochrome f interaction. Biochemistry. 1989 Oct 3;28(20):8039–8047. doi: 10.1021/bi00446a011. [DOI] [PubMed] [Google Scholar]
- Moser C. C., Keske J. M., Warncke K., Farid R. S., Dutton P. L. Nature of biological electron transfer. Nature. 1992 Feb 27;355(6363):796–802. doi: 10.1038/355796a0. [DOI] [PubMed] [Google Scholar]
- Nordling M., Olausson T., Lundberg L. G. Expression of spinach plastocyanin in E. coli. FEBS Lett. 1990 Dec 10;276(1-2):98–102. doi: 10.1016/0014-5793(90)80517-m. [DOI] [PubMed] [Google Scholar]
- Pearson D. C., Jr, Gross E. L., David E. S. Electrostatic properties of cytochrome f: implications for docking with plastocyanin. Biophys J. 1996 Jul;71(1):64–76. doi: 10.1016/S0006-3495(96)79236-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peerey L. M., Brothers H. M., 2nd, Hazzard J. T., Tollin G., Kostić N. M. Unimolecular and bimolecular oxidoreduction reactions involving diprotein complexes of cytochrome c and plastocyanin. Dependence of electron-transfer reactivity on charge and orientation of the docked metalloproteins. Biochemistry. 1991 Sep 24;30(38):9297–9304. doi: 10.1021/bi00102a023. [DOI] [PubMed] [Google Scholar]
- Peerey L. M., Kostić N. M. Oxidoreduction reactions involving the electrostatic and the covalent complex of cytochrome c and plastocyanin: importance of the protein rearrangement for the intracomplex electron-transfer reaction. Biochemistry. 1989 Feb 21;28(4):1861–1868. doi: 10.1021/bi00430a065. [DOI] [PubMed] [Google Scholar]
- Qin L., Kostić N. M. Electron-transfer reactions of cytochrome f with flavin semiquinones and with plastocyanin. Importance of protein-protein electrostatic interactions and of donor-acceptor coupling. Biochemistry. 1992 Jun 9;31(22):5145–5150. doi: 10.1021/bi00137a008. [DOI] [PubMed] [Google Scholar]
- Qin L., Kostić N. M. Enforced interaction of one molecule of plastocyanin with two molecules of cytochrome c and an electron-transfer reaction involving the hydrophobic patch on the plastocyanin surface. Biochemistry. 1996 Mar 19;35(11):3379–3386. doi: 10.1021/bi9516586. [DOI] [PubMed] [Google Scholar]
- Qin L., Kostić N. M. Importance of protein rearrangement in the electron-transfer reaction between the physiological partners cytochrome f and plastocyanin. Biochemistry. 1993 Jun 15;32(23):6073–6080. doi: 10.1021/bi00074a019. [DOI] [PubMed] [Google Scholar]
- Rich P. R., Bendall D. S. The redox potentials of the b-type cytochromes of higher plant chloroplasts. Biochim Biophys Acta. 1980 Jun 10;591(1):153–161. doi: 10.1016/0005-2728(80)90229-7. [DOI] [PubMed] [Google Scholar]
- Roberts V. A., Freeman H. C., Olson A. J., Tainer J. A., Getzoff E. D. Electrostatic orientation of the electron-transfer complex between plastocyanin and cytochrome c. J Biol Chem. 1991 Jul 15;266(20):13431–13441. [PubMed] [Google Scholar]
- Sharp K. A., Honig B. Electrostatic interactions in macromolecules: theory and applications. Annu Rev Biophys Biophys Chem. 1990;19:301–332. doi: 10.1146/annurev.bb.19.060190.001505. [DOI] [PubMed] [Google Scholar]
- Sigfridsson K., Young S., Hansson O. Structural dynamics in the plastocyanin-photosystem 1 electron-transfer complex as revealed by mutant studies. Biochemistry. 1996 Jan 30;35(4):1249–1257. doi: 10.1021/bi9520141. [DOI] [PubMed] [Google Scholar]
- Soriano G. M., Ponamarev M. V., Tae G. S., Cramer W. A. Effect of the interdomain basic region of cytochrome f on its redox reactions in vivo. Biochemistry. 1996 Nov 19;35(46):14590–14598. doi: 10.1021/bi9616211. [DOI] [PubMed] [Google Scholar]
- Takabe T., Ishikawa H. Kinetic studies on a cross-linked complex between plastocyanin cytochrome f. J Biochem. 1989 Jan;105(1):98–102. doi: 10.1093/oxfordjournals.jbchem.a122627. [DOI] [PubMed] [Google Scholar]
- Vanderkooi J., Erecińska M. Cytochrome c interaction with membranes. Interaction of cytochrome c with isolated membrane fragments and purified enzymes. Arch Biochem Biophys. 1974 Jun;162(2):385–391. doi: 10.1016/0003-9861(74)90196-9. [DOI] [PubMed] [Google Scholar]
- Wagner M. J., Packer J. C., Howe C. J., Bendall D. S. Some characteristics of cytochrome f in the cyanobacterium Phormidium laminosum: its sequence and charge properties in the reaction with plastocyanin. Biochim Biophys Acta. 1996 Sep 30;1276(3):246–252. doi: 10.1016/0005-2728(96)00084-9. [DOI] [PubMed] [Google Scholar]
- Zhang H., Carrell C. J., Huang D., Sled V., Ohnishi T., Smith J. L., Cramer W. A. Characterization and crystallization of the lumen side domain of the chloroplast Rieske iron-sulfur protein. J Biol Chem. 1996 Dec 6;271(49):31360–31366. doi: 10.1074/jbc.271.49.31360. [DOI] [PubMed] [Google Scholar]


