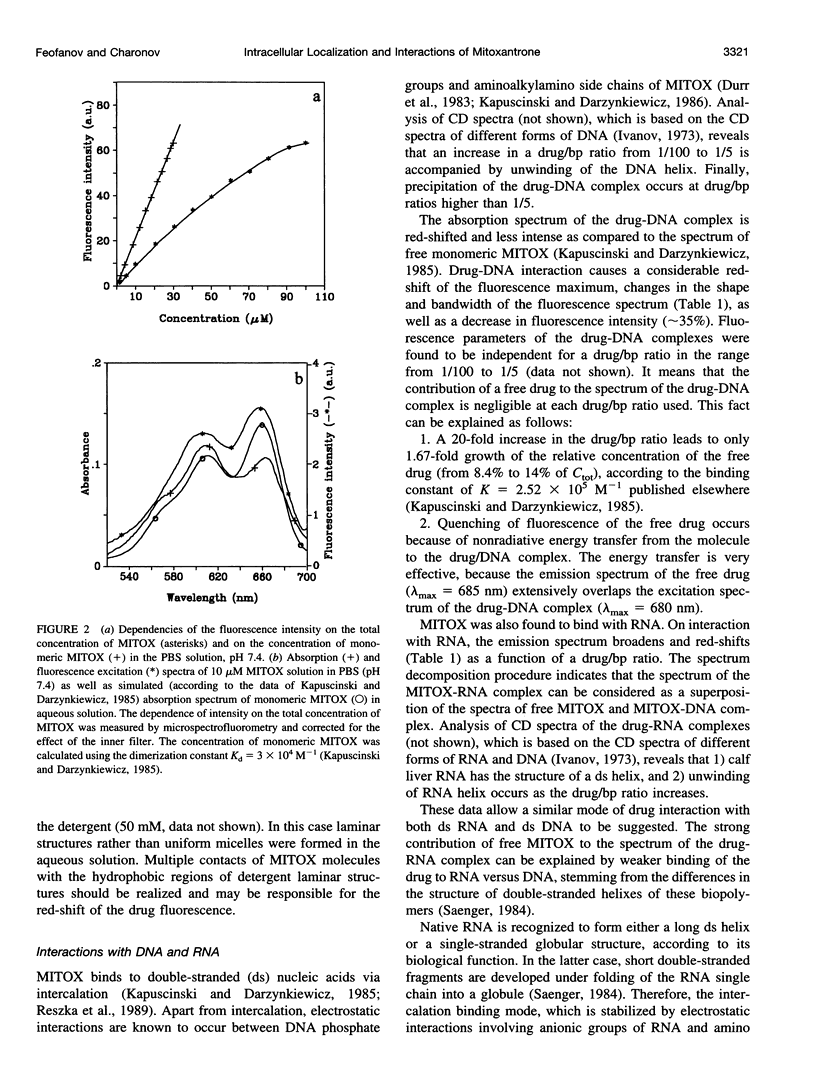
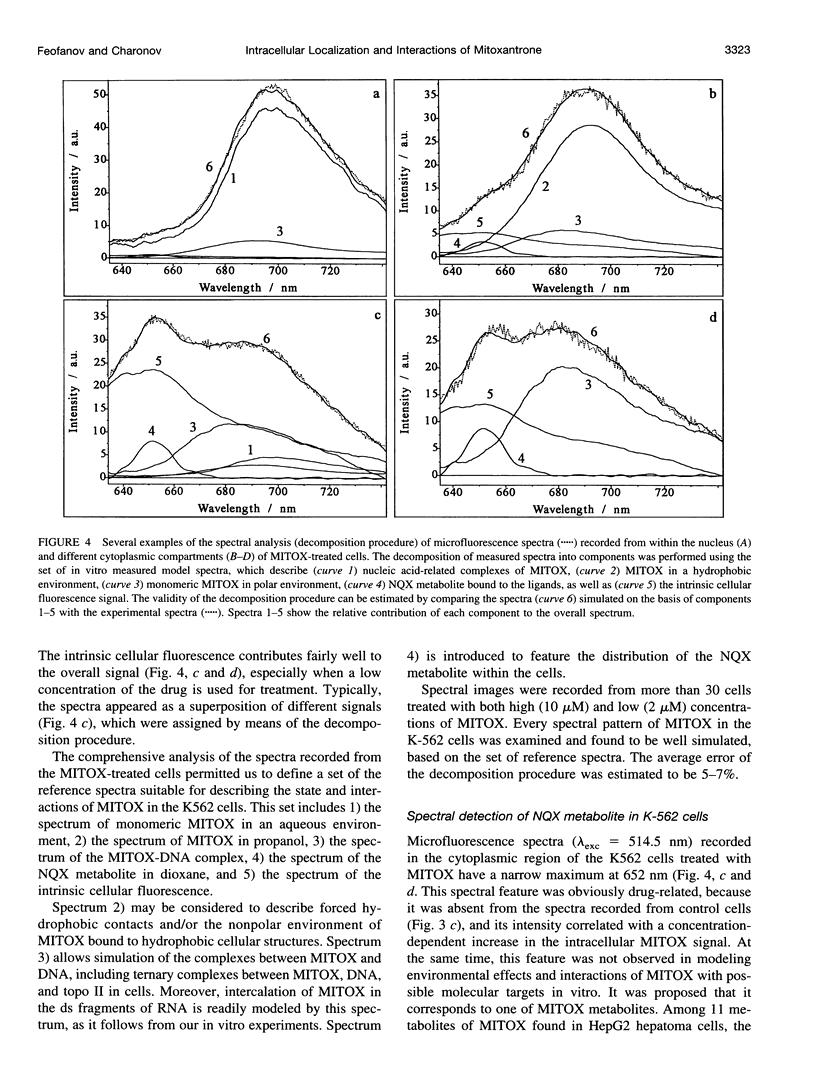
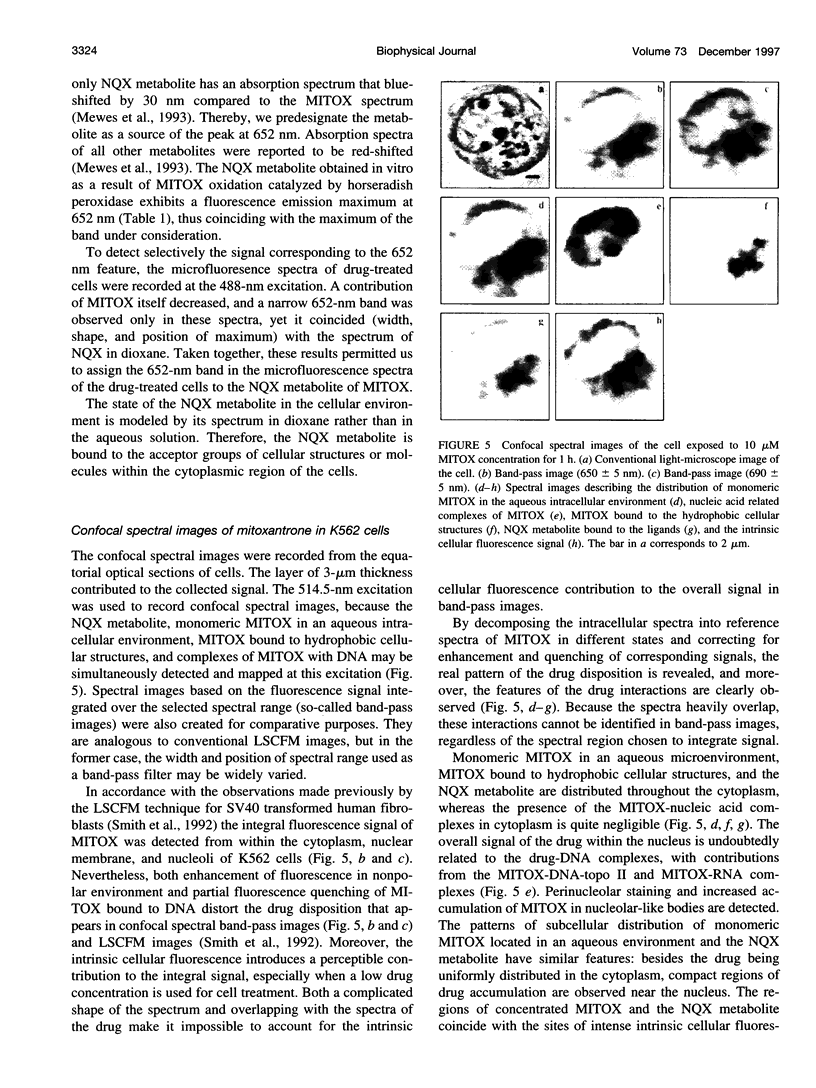
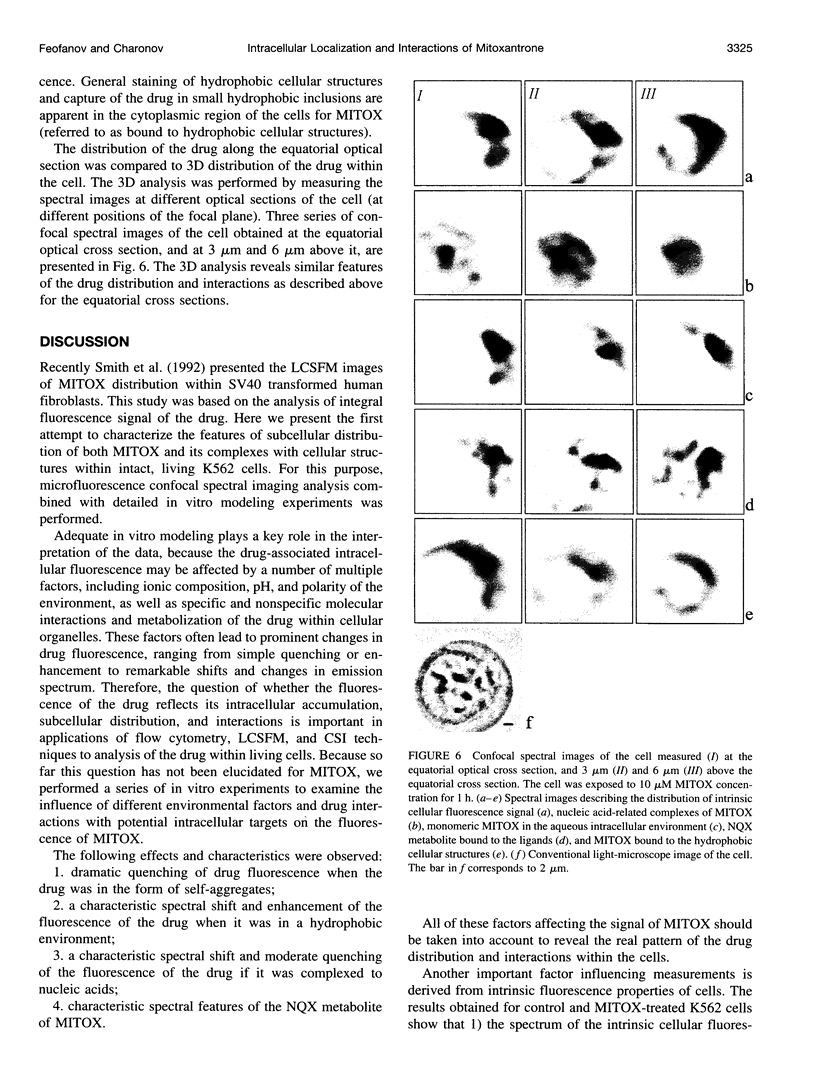
Abstract
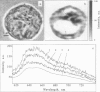
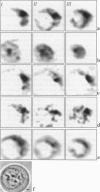
Studying mechanisms of drug antitumor action is complicated by the lack of noninvasive methods enabling direct monitoring of the state and interactions of the drugs within intact viable cells. Here we present a confocal spectral imaging (CSI) technique as a method of overcoming this problem. We applied this method to the examination of localization and interactions of mitoxantrone (1, 4-dihydroxy-5, 8-bis-[([2-(2-hydroxyethyl)-amino]ethyl)amino]-9,10-anthracenedione dihydrochloride), a potent antitumor drug, in living K562 cells. A two-dimensional set of fluorescence spectra of mitoxantrone (MITOX) recorded with micron resolution within a drug-treated cell was analyzed to reveal formation of drug-target complexes and to create the maps of their intracellular distribution. The analysis was based on detailed in vitro modeling of drug-target (DNA, RNA, DNA topoisomerase II) interactions and environmental effects affecting drug fluorescence. MITOX exposed to aqueous intracellular environment, MITOX bound to hydrophobic cellular structures, complexes of MITOX with nucleic acids, as well as the naphtoquinoxaline metabolite of MITOX were simultaneously detected and mapped in K562 cells. These states and complexes are known to be immediately related to the antitumor action of the drug. The results obtained present a basis for the subsequent quantitative analysis of concentration and time-dependent accumulation of free and bound MITOX within different compartments of living cancer cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arlin Z., Case D. C., Jr, Moore J., Wiernik P., Feldman E., Saletan S., Desai P., Sia L., Cartwright K. Randomized multicenter trial of cytosine arabinoside with mitoxantrone or daunorubicin in previously untreated adult patients with acute nonlymphocytic leukemia (ANLL). Lederle Cooperative Group. Leukemia. 1990 Mar;4(3):177–183. [PubMed] [Google Scholar]
- D'Arpa P., Liu L. F. Topoisomerase-targeting antitumor drugs. Biochim Biophys Acta. 1989 Dec 17;989(2):163–177. doi: 10.1016/0304-419x(89)90041-3. [DOI] [PubMed] [Google Scholar]
- Durr F. E., Wallace R. E., Citarella R. V. Molecular and biochemical pharmacology of mitoxantrone. Cancer Treat Rev. 1983 Dec;10 (Suppl B):3–11. doi: 10.1016/0305-7372(83)90016-6. [DOI] [PubMed] [Google Scholar]
- Ehninger G., Schuler U., Proksch B., Zeller K. P., Blanz J. Pharmacokinetics and metabolism of mitoxantrone. A review. Clin Pharmacokinet. 1990 May;18(5):365–380. doi: 10.2165/00003088-199018050-00003. [DOI] [PubMed] [Google Scholar]
- Faulds D., Balfour J. A., Chrisp P., Langtry H. D. Mitoxantrone. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in the chemotherapy of cancer. Drugs. 1991 Mar;41(3):400–449. doi: 10.2165/00003495-199141030-00007. [DOI] [PubMed] [Google Scholar]
- Fujimoto S., Ogawa M. Antitumor activity of mitoxantrone against murine experimental tumors: comparative analysis against various antitumor antibiotics. Cancer Chemother Pharmacol. 1982;8(2):157–162. doi: 10.1007/BF00255476. [DOI] [PubMed] [Google Scholar]
- Gigli M., Doglia S. M., Millot J. M., Valentini L., Manfait M. Quantitative study of doxorubicin in living cell nuclei by microspectrofluorometry. Biochim Biophys Acta. 1988 May 6;950(1):13–20. doi: 10.1016/0167-4781(88)90068-1. [DOI] [PubMed] [Google Scholar]
- Ho C. K., Law S. L., Chiang H., Hsu M. L., Wang C. C., Wang S. Y. Inhibition of microtubule assembly is a possible mechanism of action of mitoxantrone. Biochem Biophys Res Commun. 1991 Oct 15;180(1):118–123. doi: 10.1016/s0006-291x(05)81263-x. [DOI] [PubMed] [Google Scholar]
- Johnson R. K., Zee-Cheng R. K., Lee W. W., Acton E. M., Henry D. W., Cheng C. C. Experimental antitumor activity of aminoanthraquinones. Cancer Treat Rep. 1979 Mar;63(3):425–439. [PubMed] [Google Scholar]
- Kapuscinski J., Darzynkiewicz Z. Interactions of antitumor agents Ametantrone and Mitoxantrone (Novatrone) with double-stranded DNA. Biochem Pharmacol. 1985 Dec 15;34(24):4203–4213. doi: 10.1016/0006-2952(85)90275-8. [DOI] [PubMed] [Google Scholar]
- Kapuscinski J., Darzynkiewicz Z. Relationship between the pharmacological activity of antitumor drugs Ametantrone and mitoxantrone (Novatrone) and their ability to condense nucleic acids. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6302–6306. doi: 10.1073/pnas.83.17.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapuscinski J., Darzynkiewicz Z., Traganos F., Melamed M. R. Interactions of a new antitumor agent, 1,4-dihydroxy-5,8-bis[[2-[(2-hydroxyethyl)amino]-ethyl]amino]-9,10-anthracenedione, with nucleic acids. Biochem Pharmacol. 1981 Feb 1;30(3):231–240. doi: 10.1016/0006-2952(81)90083-6. [DOI] [PubMed] [Google Scholar]
- Liyanage M., Coleman A., du Manoir S., Veldman T., McCormack S., Dickson R. B., Barlow C., Wynshaw-Boris A., Janz S., Wienberg J. Multicolour spectral karyotyping of mouse chromosomes. Nat Genet. 1996 Nov;14(3):312–315. doi: 10.1038/ng1196-312. [DOI] [PubMed] [Google Scholar]
- Malik Z., Dishi M., Garini Y. Fourier transform multipixel spectroscopy and spectral imaging of protoporphyrin in single melanoma cells. Photochem Photobiol. 1996 May;63(5):608–614. doi: 10.1111/j.1751-1097.1996.tb05663.x. [DOI] [PubMed] [Google Scholar]
- Mewes K., Blanz J., Ehninger G., Gebhardt R., Zeller K. P. Cytochrome P-450-induced cytotoxicity of mitoxantrone by formation of electrophilic intermediates. Cancer Res. 1993 Nov 1;53(21):5135–5142. [PubMed] [Google Scholar]
- Nabiev I., Chourpa I., Riou J. F., Nguyen C. H., Lavelle F., Manfait M. Molecular interactions of DNA-topoisomerase I and II inhibitor with DNA and topoisomerases and in ternary complexes: binding modes and biological effects for intoplicine derivatives. Biochemistry. 1994 Aug 2;33(30):9013–9023. doi: 10.1021/bi00196a020. [DOI] [PubMed] [Google Scholar]
- Reszka K., Hartley J. A., Kolodziejczyk P., Lown J. W. Interaction of the peroxidase-derived metabolite of mitoxantrone with nucleic acids. Evidence for covalent binding of 14C-labeled drug. Biochem Pharmacol. 1989 Dec 1;38(23):4253–4260. doi: 10.1016/0006-2952(89)90523-6. [DOI] [PubMed] [Google Scholar]
- Reszka K., Kolodziejczyk P., Lown J. W. Horseradish peroxidase-catalyzed oxidation of mitoxantrone: spectrophotometric and electron paramagnetic resonance studies. J Free Radic Biol Med. 1986;2(1):25–32. doi: 10.1016/0748-5514(86)90120-0. [DOI] [PubMed] [Google Scholar]
- Roberts R. A., Cress A. E., Dalton W. S. Persistent intracellular binding of mitoxantrone in a human colon carcinoma cell line. Biochem Pharmacol. 1989 Dec 1;38(23):4283–4290. doi: 10.1016/0006-2952(89)90527-3. [DOI] [PubMed] [Google Scholar]
- Smith P. J., Morgan S. A., Fox M. E., Watson J. V. Mitoxantrone-DNA binding and the induction of topoisomerase II associated DNA damage in multi-drug resistant small cell lung cancer cells. Biochem Pharmacol. 1990 Nov 1;40(9):2069–2078. doi: 10.1016/0006-2952(90)90237-f. [DOI] [PubMed] [Google Scholar]
- Smith P. J., Sykes H. R., Fox M. E., Furlong I. J. Subcellular distribution of the anticancer drug mitoxantrone in human and drug-resistant murine cells analyzed by flow cytometry and confocal microscopy and its relationship to the induction of DNA damage. Cancer Res. 1992 Jul 15;52(14):4000–4008. [PubMed] [Google Scholar]