Abstract
Confocal spectral imaging (CSI) technique was used for quantitative analysis of the uptake, subcellular localization, and characteristics of localized binding and retention of anticancer agent mitoxantrone (MITOX) within human K562 erythroleukemia cells. The CSI technique enables identification of the state and interactions of the drug within the living cells. Utilizing this unique property of the method, intracellular distributions were examined for monomeric MITOX in polar environment, MITOX bound with hydrophobic cellular structures, naphthoquinoxaline metabolite, and nucleic acid-related complexes of MITOX. The features revealed were compared for the cells treated with 2 microM or 10 microM of MITOX for 1 h and correlated to the known data on antitumor action of the drug. MITOX was found to exhibit high tendency to self-aggregation within intracellular media. The aggregates are concluded to be a determinant of long-term intracellular retention of the drug and a source of persistent intracellular binding of MITOX. Considerable penetration of MITOX in the hydrophobic cytoskeleton structures as well as growing accumulation of MITOX bound to nucleic acids within the nucleus were found to occur in the cells treated with a high concentration of the drug. These effects may be among the factors stimulating and/or accompanying high-dose mitoxantrone-induced programmed cell death or apoptosis.
Full text
PDF

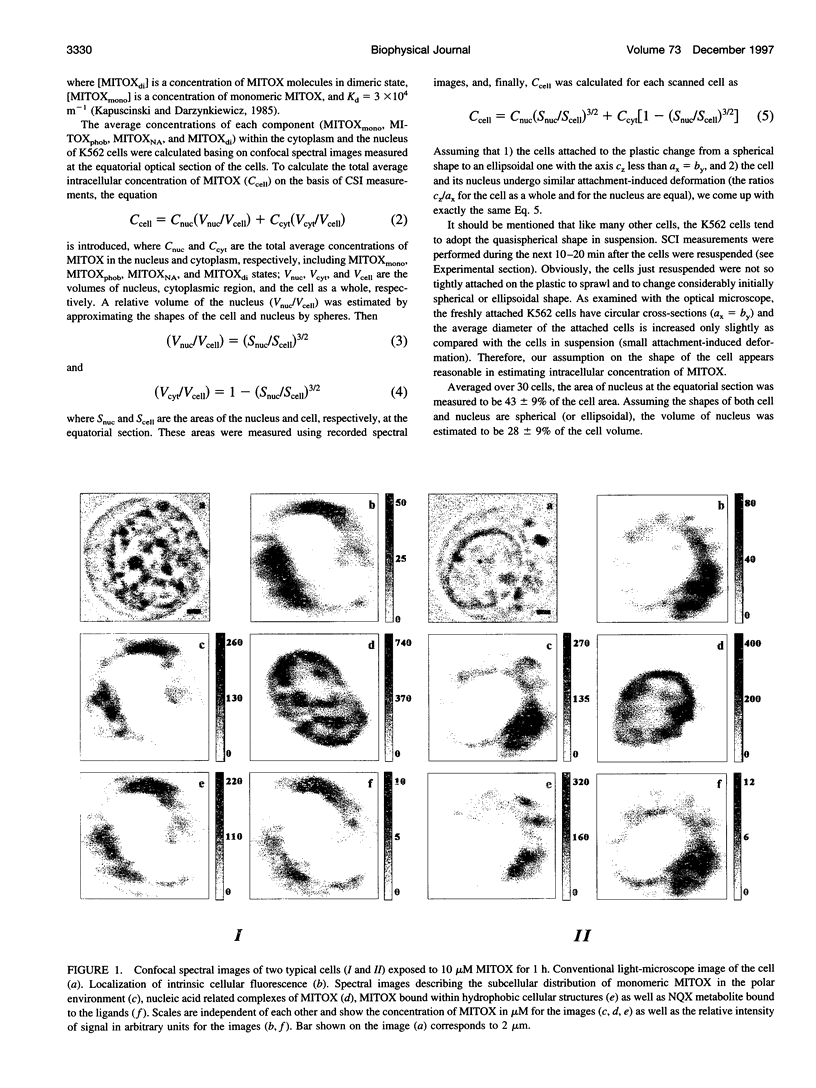
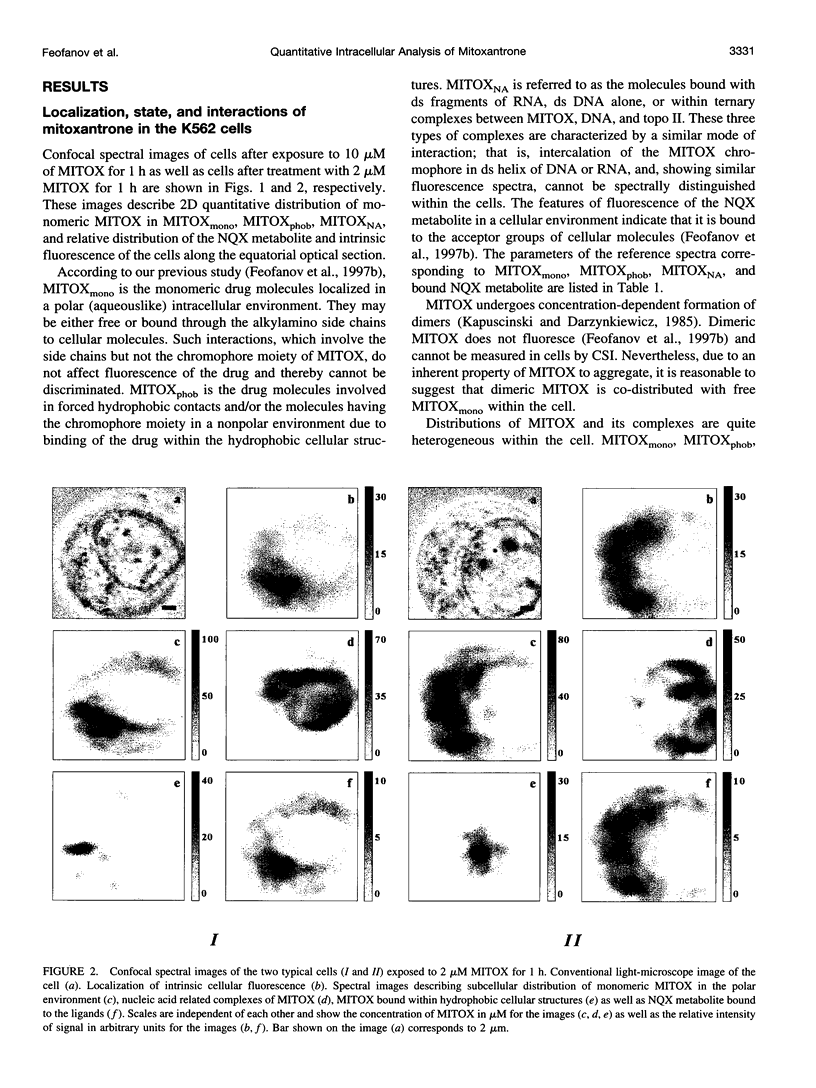




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arlin Z., Case D. C., Jr, Moore J., Wiernik P., Feldman E., Saletan S., Desai P., Sia L., Cartwright K. Randomized multicenter trial of cytosine arabinoside with mitoxantrone or daunorubicin in previously untreated adult patients with acute nonlymphocytic leukemia (ANLL). Lederle Cooperative Group. Leukemia. 1990 Mar;4(3):177–183. [PubMed] [Google Scholar]
- Basra J., Wolf C. R., Brown J. R., Patterson L. H. Evidence for human liver mediated free-radical formation by doxorubicin and mitozantrone. Anticancer Drug Des. 1985 Oct;1(1):45–52. [PubMed] [Google Scholar]
- Bhalla K., Ibrado A. M., Tourkina E., Tang C., Grant S., Bullock G., Huang Y., Ponnathpur V., Mahoney M. E. High-dose mitoxantrone induces programmed cell death or apoptosis in human myeloid leukemia cells. Blood. 1993 Nov 15;82(10):3133–3140. [PubMed] [Google Scholar]
- Blanz J., Mewes K., Ehninger G., Proksch B., Waidelich D., Greger B., Zeller K. P. Evidence for oxidative activation of mitoxantrone in human, pig, and rat. Drug Metab Dispos. 1991 Sep-Oct;19(5):871–880. [PubMed] [Google Scholar]
- Corbett A. H., Osheroff N. When good enzymes go bad: conversion of topoisomerase II to a cellular toxin by antineoplastic drugs. Chem Res Toxicol. 1993 Sep-Oct;6(5):585–597. doi: 10.1021/tx00035a001. [DOI] [PubMed] [Google Scholar]
- D'Arpa P., Liu L. F. Topoisomerase-targeting antitumor drugs. Biochim Biophys Acta. 1989 Dec 17;989(2):163–177. doi: 10.1016/0304-419x(89)90041-3. [DOI] [PubMed] [Google Scholar]
- Ehninger G., Proksch B., Heinzel G., Woodward D. L. Clinical pharmacology of mitoxantrone. Cancer Treat Rep. 1986 Dec;70(12):1373–1378. [PubMed] [Google Scholar]
- Ehninger G., Schuler U., Proksch B., Zeller K. P., Blanz J. Pharmacokinetics and metabolism of mitoxantrone. A review. Clin Pharmacokinet. 1990 May;18(5):365–380. doi: 10.2165/00003088-199018050-00003. [DOI] [PubMed] [Google Scholar]
- Faulds D., Balfour J. A., Chrisp P., Langtry H. D. Mitoxantrone. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in the chemotherapy of cancer. Drugs. 1991 Mar;41(3):400–449. doi: 10.2165/00003495-199141030-00007. [DOI] [PubMed] [Google Scholar]
- Feofanov A., Sharonov S., Kudelina I., Fleury F., Nabiev I. Localization and molecular interactions of mitoxantrone within living K562 cells as probed by confocal spectral imaging analysis. Biophys J. 1997 Dec;73(6):3317–3327. doi: 10.1016/S0006-3495(97)78356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M. E., Smith P. J. Long-term inhibition of DNA synthesis and the persistence of trapped topoisomerase II complexes in determining the toxicity of the antitumor DNA intercalators mAMSA and mitoxantrone. Cancer Res. 1990 Sep 15;50(18):5813–5818. [PubMed] [Google Scholar]
- Ho C. K., Law S. L., Chiang H., Hsu M. L., Wang C. C., Wang S. Y. Inhibition of microtubule assembly is a possible mechanism of action of mitoxantrone. Biochem Biophys Res Commun. 1991 Oct 15;180(1):118–123. doi: 10.1016/s0006-291x(05)81263-x. [DOI] [PubMed] [Google Scholar]
- Kapuscinski J., Darzynkiewicz Z. Interactions of antitumor agents Ametantrone and Mitoxantrone (Novatrone) with double-stranded DNA. Biochem Pharmacol. 1985 Dec 15;34(24):4203–4213. doi: 10.1016/0006-2952(85)90275-8. [DOI] [PubMed] [Google Scholar]
- Kapuscinski J., Darzynkiewicz Z. Relationship between the pharmacological activity of antitumor drugs Ametantrone and mitoxantrone (Novatrone) and their ability to condense nucleic acids. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6302–6306. doi: 10.1073/pnas.83.17.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapuscinski J., Darzynkiewicz Z., Traganos F., Melamed M. R. Interactions of a new antitumor agent, 1,4-dihydroxy-5,8-bis[[2-[(2-hydroxyethyl)amino]-ethyl]amino]-9,10-anthracenedione, with nucleic acids. Biochem Pharmacol. 1981 Feb 1;30(3):231–240. doi: 10.1016/0006-2952(81)90083-6. [DOI] [PubMed] [Google Scholar]
- Kolodziejczyk P., Reszka K., Lown J. W. Enzymatic oxidative activation and transformation of the antitumor agent mitoxantrone. Free Radic Biol Med. 1988;5(1):13–25. doi: 10.1016/0891-5849(88)90058-5. [DOI] [PubMed] [Google Scholar]
- Maneshin S. K., Arevshatian A. A. Liuinestsentsiia profirinov v drozhzhakh p. Candida, vyrashchivaemykh na uglevodorodakh. Biofizika. 1972 Mar-Apr;17(2):352–354. [PubMed] [Google Scholar]
- Mewes K., Blanz J., Ehninger G., Gebhardt R., Zeller K. P. Cytochrome P-450-induced cytotoxicity of mitoxantrone by formation of electrophilic intermediates. Cancer Res. 1993 Nov 1;53(21):5135–5142. [PubMed] [Google Scholar]
- Reszka K., Kolodziejczyk P., Lown J. W. Horseradish peroxidase-catalyzed oxidation of mitoxantrone: spectrophotometric and electron paramagnetic resonance studies. J Free Radic Biol Med. 1986;2(1):25–32. doi: 10.1016/0748-5514(86)90120-0. [DOI] [PubMed] [Google Scholar]
- Roberts R. A., Cress A. E., Dalton W. S. Persistent intracellular binding of mitoxantrone in a human colon carcinoma cell line. Biochem Pharmacol. 1989 Dec 1;38(23):4283–4290. doi: 10.1016/0006-2952(89)90527-3. [DOI] [PubMed] [Google Scholar]
- Smith P. J., Morgan S. A., Fox M. E., Watson J. V. Mitoxantrone-DNA binding and the induction of topoisomerase II associated DNA damage in multi-drug resistant small cell lung cancer cells. Biochem Pharmacol. 1990 Nov 1;40(9):2069–2078. doi: 10.1016/0006-2952(90)90237-f. [DOI] [PubMed] [Google Scholar]
- Smith P. J., Sykes H. R., Fox M. E., Furlong I. J. Subcellular distribution of the anticancer drug mitoxantrone in human and drug-resistant murine cells analyzed by flow cytometry and confocal microscopy and its relationship to the induction of DNA damage. Cancer Res. 1992 Jul 15;52(14):4000–4008. [PubMed] [Google Scholar]
- Wallace R. E., Lindh D., Durr F. E. Development of resistance and characteristics of a human colon carcinoma subline resistant to mitoxantrone in vitro. Cancer Invest. 1987;5(5):417–428. doi: 10.3109/07357908709032899. [DOI] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1996;65:635–692. doi: 10.1146/annurev.bi.65.070196.003223. [DOI] [PubMed] [Google Scholar]
- Wolf C. R., Macpherson J. S., Smyth J. F. Evidence for the metabolism of mitozantrone by microsomal glutathione transferases and 3-methylcholanthrene-inducible glucuronosyl transferases. Biochem Pharmacol. 1986 May 1;35(9):1577–1581. doi: 10.1016/0006-2952(86)90127-9. [DOI] [PubMed] [Google Scholar]
- Zini N., Santi S., Ognibene A., Bavelloni A., Neri L. M., Valmori A., Mariani E., Negri C., Astaldi-Ricotti G. C., Maraldi N. M. Discrete localization of different DNA topoisomerases in HeLa and K562 cell nuclei and subnuclear fractions. Exp Cell Res. 1994 Feb;210(2):336–348. doi: 10.1006/excr.1994.1046. [DOI] [PubMed] [Google Scholar]