Abstract
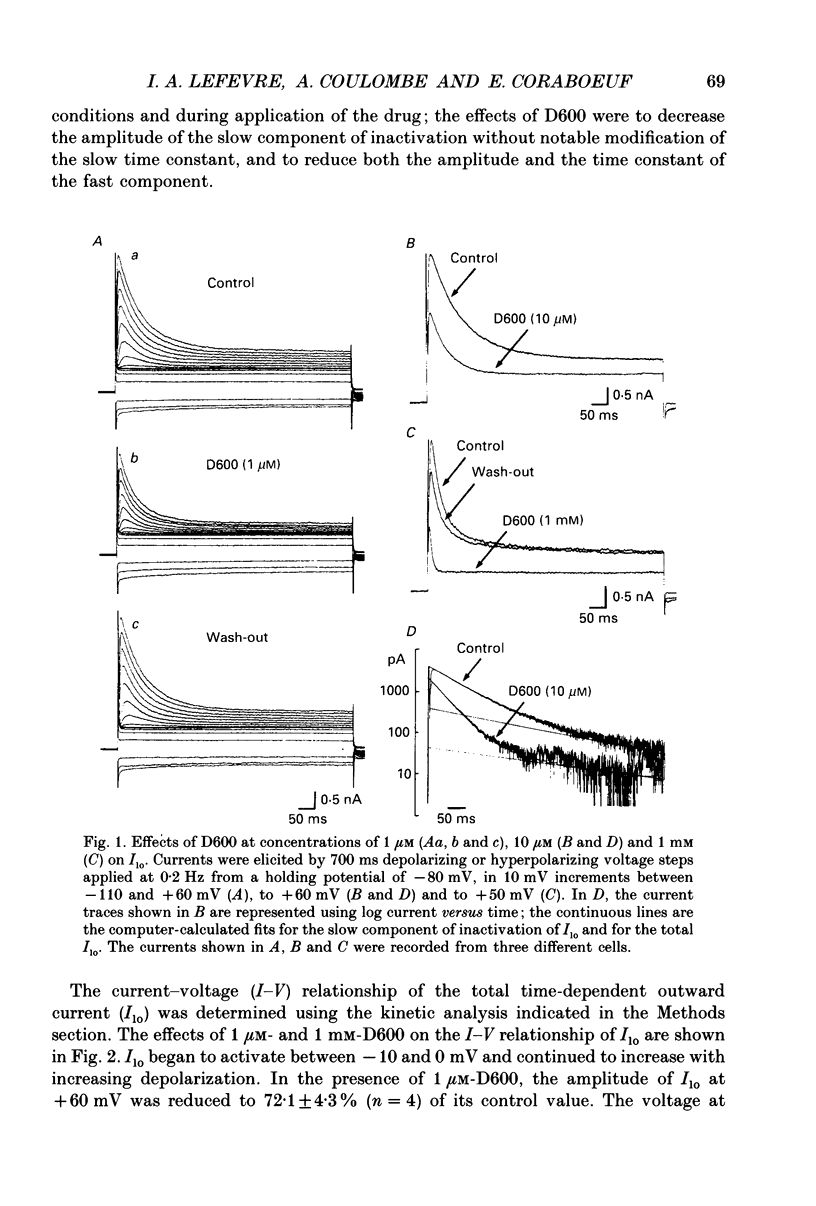
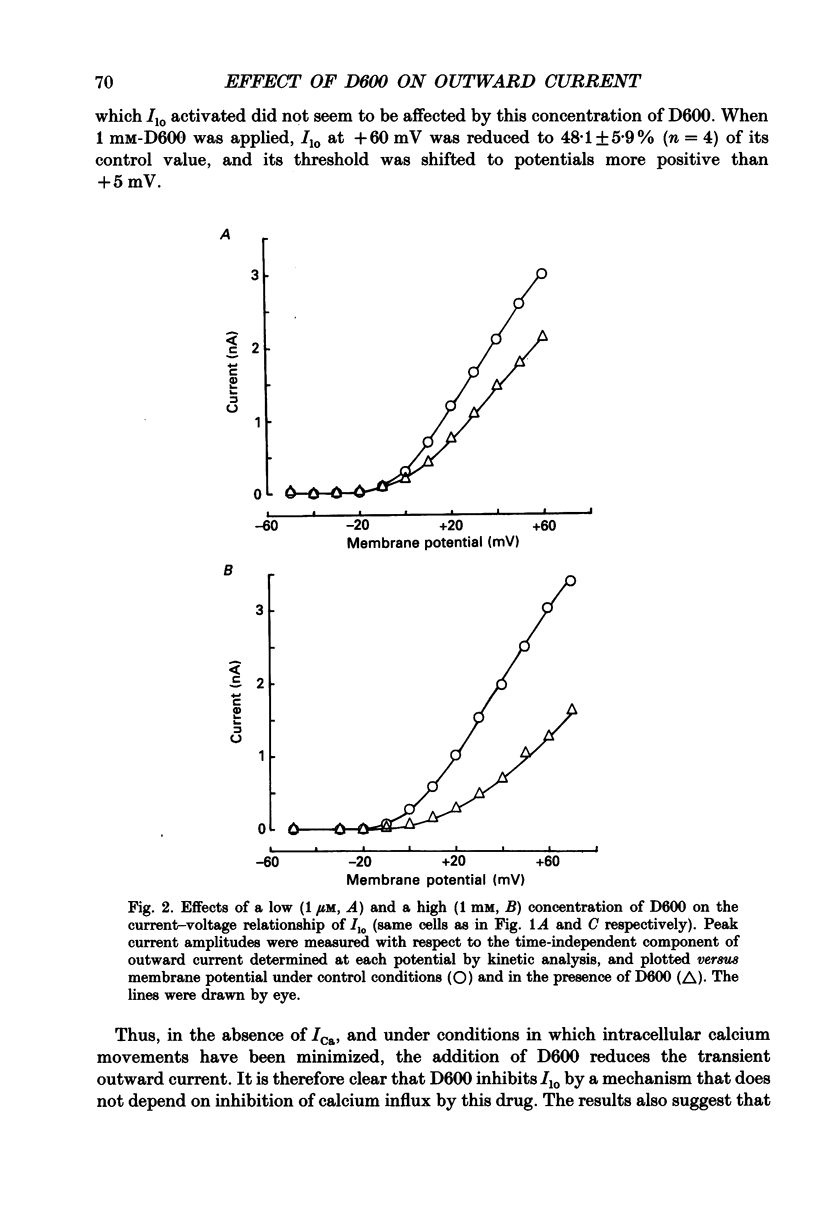
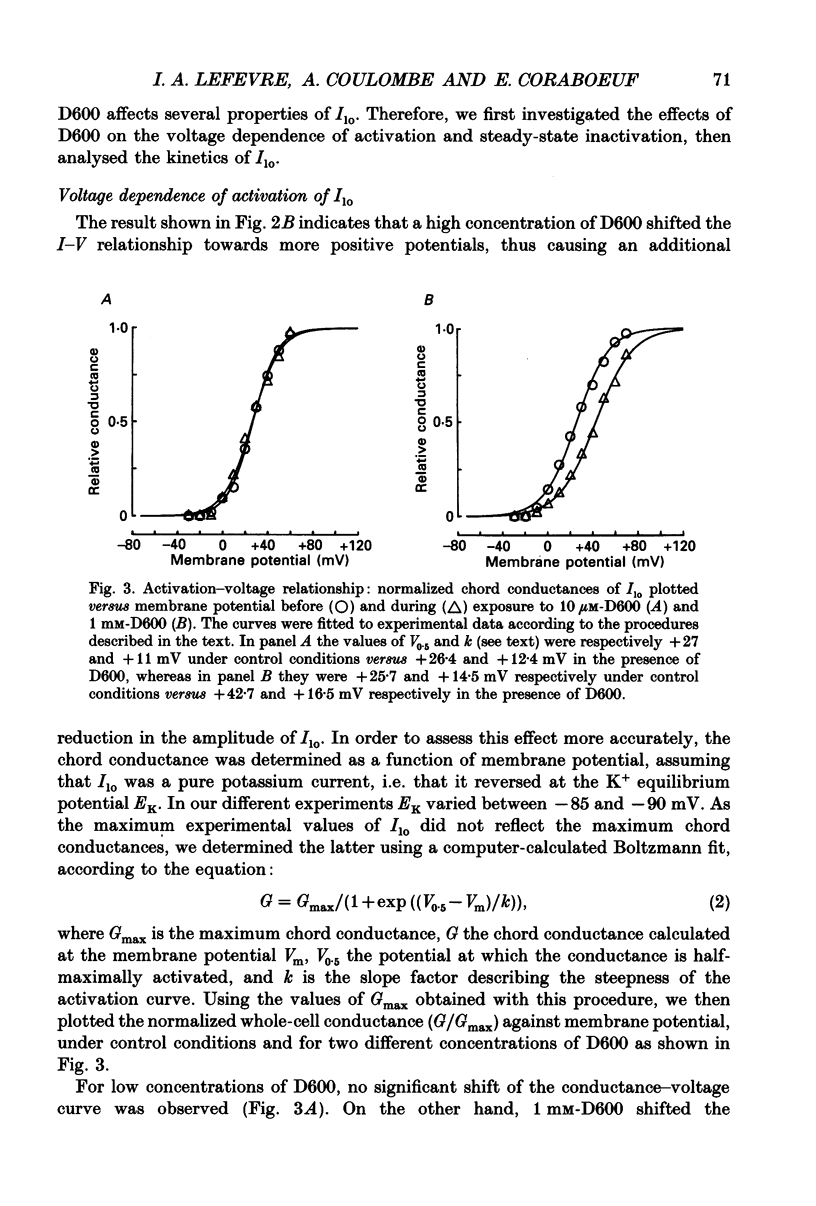
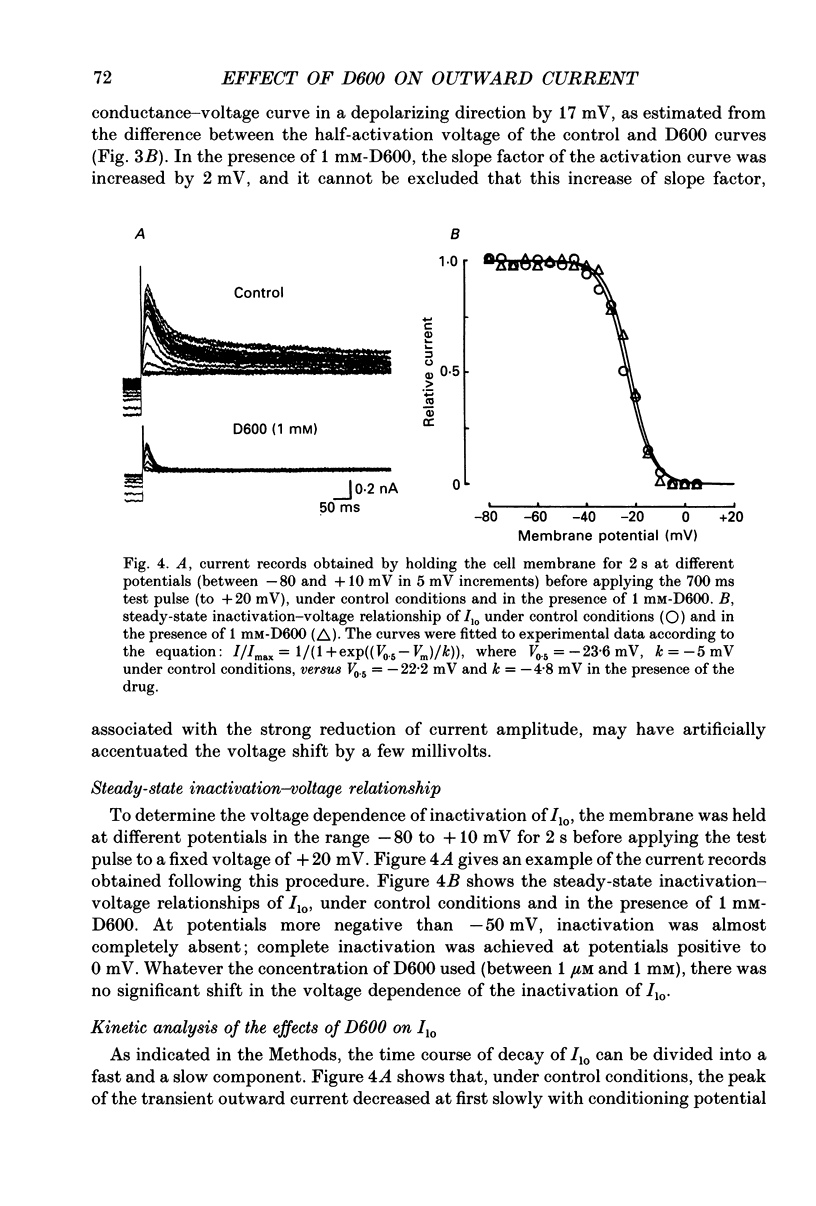
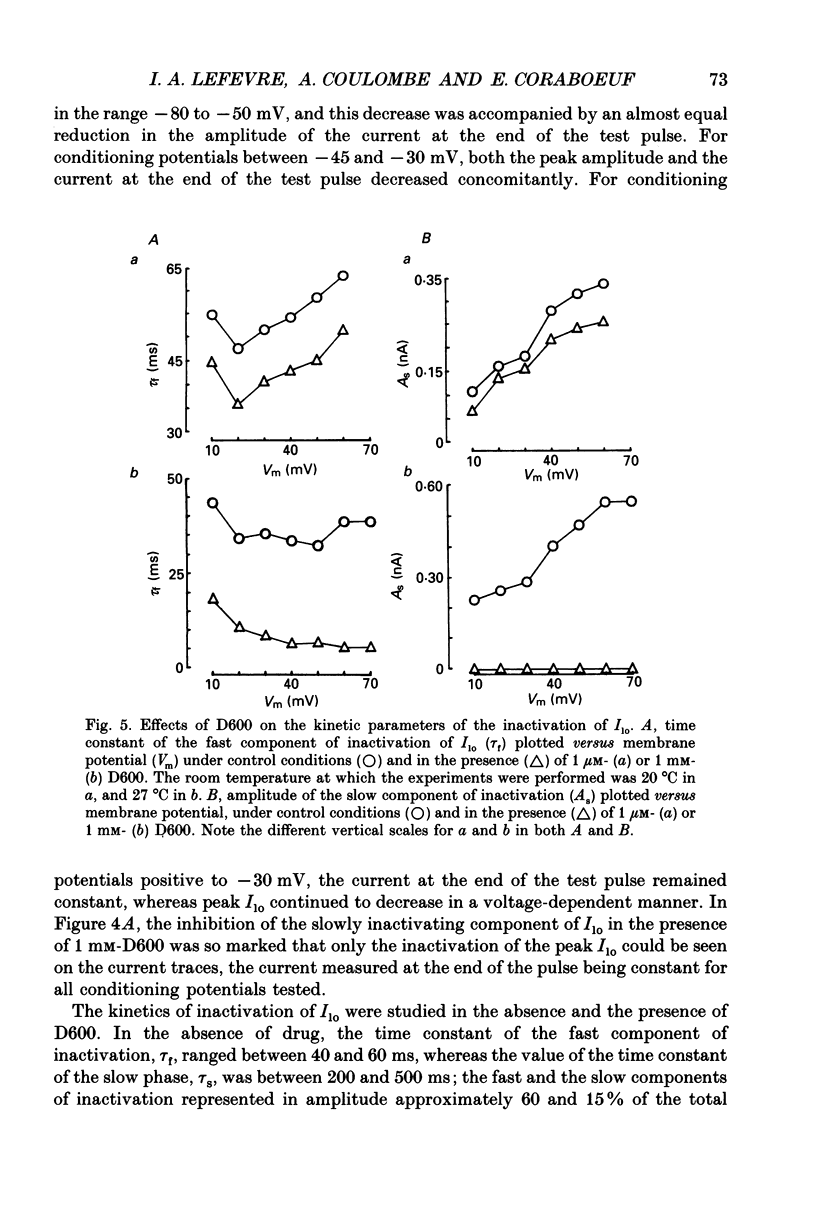
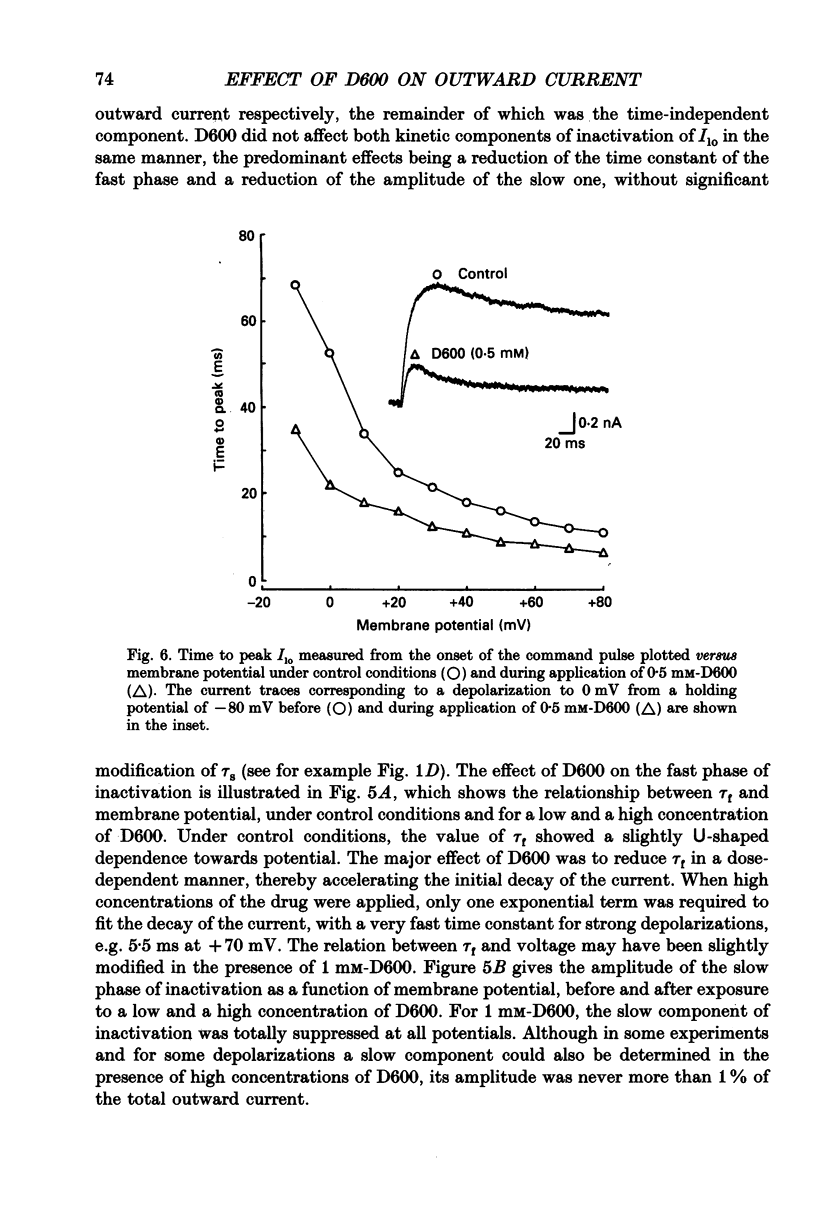
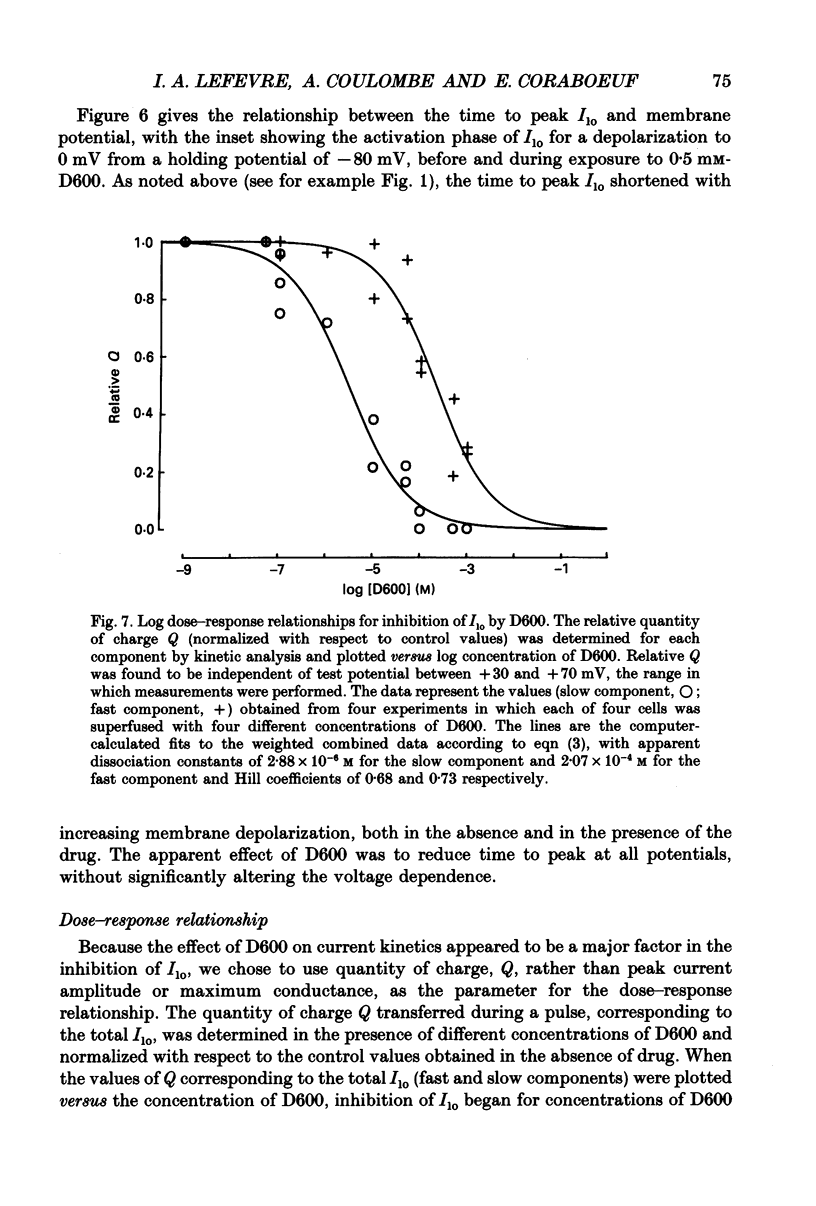
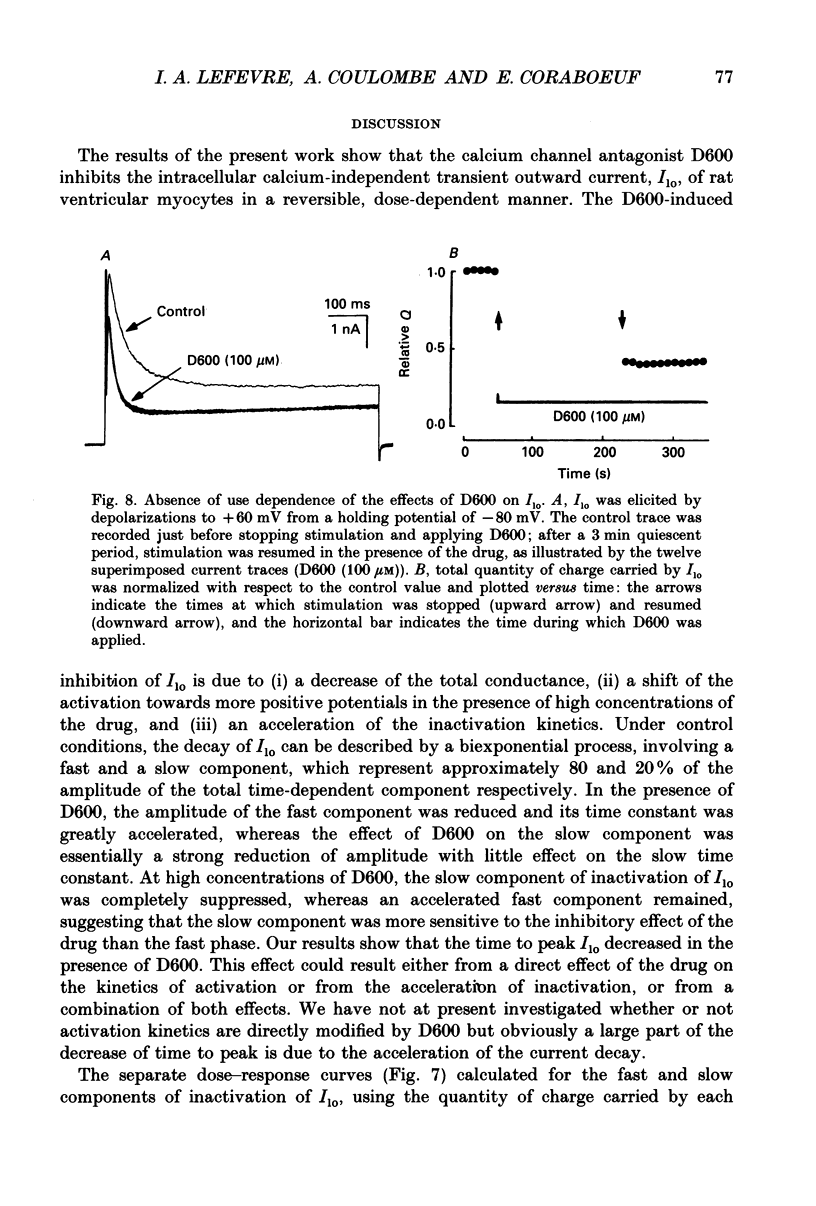
1. The whole-cell voltage-clamp technique was applied to isolated rat ventricular myocytes to investigate the effects of D600 (10(-9)-10(-3) M) on the intracellular calcium-independent component of transient outward current. I(lo), recorded in a sodium-free medium containing 0.5 x 10(-3) M-cadmium and 10(-6) M-ryanodine. 2. Externally applied D600 reduced Ilo in a dose-dependent, reversible manner, and accelerated the decay of the current. 3. Current-voltage relationships and corresponding activation curves (determined assuming I(lo) to be a pure potassium current) were shifted towards positive potentials in the presence of 10(-3) M but not 10(-5) M-D600. Steady-state inactivation curves were not affected by either low or high concentrations of D600. 4. Under control conditions, the inactivation of I(lo) is composed of a fast and a slow component. The amplitude of the slow component was more strongly reduced by D600 than that of the fast one. In the presence of 10(-3) M-D600, the slow component was entirely suppressed. 5. Both the time to peak Ilo and the time constant of the fast component of inactivation were markedly reduced at all potentials by D600. The time constant of the slow component was less sensitive to the drug. 6. When the relative quantity of charge carried by each kinetic component of Ilo was plotted versus the concentration of D600, the data could be fitted by two distinctly separate dose-response curves with an almost 100-fold difference between the two apparent dissociation constants, of which the values were 2.88 x 10(-6) M for the slow phase of inactivation and 2.07 x 10(-4) M for the fast one, with Hill coefficients of 0.68 and 0.73 respectively. 7. The inhibition of I(lo) by D600 displayed little or no use dependence, one of the major characteristics of the effects of phenylalkylamines on the cardiac calcium current ICa. 8. Our results show that at least part of I(lo) is sensitive to D600 in the same range of concentrations as ICa. Although the effects of D600 on the two currents differ in several points, this observation raises the possibility that, besides clear differences, certain similarities may exist between the channels responsible for I(lo) and ICa.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apkon M., Nerbonne J. M. Alpha 1-adrenergic agonists selectively suppress voltage-dependent K+ current in rat ventricular myocytes. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8756–8760. doi: 10.1073/pnas.85.22.8756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benndorf K., Nilius B. Different blocking effects of Cd++ and Hg++ on the early outward current in myocardial mouse cells. Gen Physiol Biophys. 1988 Aug;7(4):345–351. [PubMed] [Google Scholar]
- Berger F., Borchard U., Hafner D. Effects of the calcium entry blocker bepridil on repolarizing and pacemaker currents in sheep cardiac Purkinje fibres. Naunyn Schmiedebergs Arch Pharmacol. 1989 Jun;339(6):638–646. doi: 10.1007/BF00168656. [DOI] [PubMed] [Google Scholar]
- Bustamante J. O. Block of sodium currents by the calcium antagonist D600 in human heart cell segments. Pflugers Arch. 1985 Feb;403(2):225–227. doi: 10.1007/BF00584106. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. Voltage-dependent block by tetrodotoxin of the sodium channel in rabbit cardiac Purkinje fibers. Biophys J. 1987 Jan;51(1):109–114. doi: 10.1016/S0006-3495(87)83315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. Structure and function of voltage-sensitive ion channels. Science. 1988 Oct 7;242(4875):50–61. doi: 10.1126/science.2459775. [DOI] [PubMed] [Google Scholar]
- Cohen N. M., Lederer W. J. Calcium current in isolated neonatal rat ventricular myocytes. J Physiol. 1987 Oct;391:169–191. doi: 10.1113/jphysiol.1987.sp016732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coraboeuf E., Carmeliet E. Existence of two transient outward currents in sheep cardiac Purkinje fibers. Pflugers Arch. 1982 Feb;392(4):352–359. doi: 10.1007/BF00581631. [DOI] [PubMed] [Google Scholar]
- Coraboeuf E., Deroubaix E., Escande D., Coulombe A. Comparative effects of three class I antiarrhythmic drugs on plateau and pacemaker currents of sheep cardiac Purkinje fibres. Cardiovasc Res. 1988 Jun;22(6):375–384. doi: 10.1093/cvr/22.6.375. [DOI] [PubMed] [Google Scholar]
- Dukes I. D., Morad M. Tedisamil inactivates transient outward K+ current in rat ventricular myocytes. Am J Physiol. 1989 Nov;257(5 Pt 2):H1746–H1749. doi: 10.1152/ajpheart.1989.257.5.H1746. [DOI] [PubMed] [Google Scholar]
- Escande D., Coulombe A., Faivre J. F., Deroubaix E., Coraboeuf E. Two types of transient outward currents in adult human atrial cells. Am J Physiol. 1987 Jan;252(1 Pt 2):H142–H148. doi: 10.1152/ajpheart.1987.252.1.H142. [DOI] [PubMed] [Google Scholar]
- Fedida D., Shimoni Y., Giles W. R. A novel effect of norepinephrine on cardiac cells is mediated by alpha 1-adrenoceptors. Am J Physiol. 1989 May;256(5 Pt 2):H1500–H1504. doi: 10.1152/ajpheart.1989.256.5.H1500. [DOI] [PubMed] [Google Scholar]
- Giles W. R., Imaizumi Y. Comparison of potassium currents in rabbit atrial and ventricular cells. J Physiol. 1988 Nov;405:123–145. doi: 10.1113/jphysiol.1988.sp017325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L., Kenyon J. L. Delays in inactivation development and activation kinetics in myxicola giant axons. J Gen Physiol. 1982 Jul;80(1):83–102. doi: 10.1085/jgp.80.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene R. W., Haas H. L., Reiner P. B. Two transient outward currents in histamine neurones of the rat hypothalamus in vitro. J Physiol. 1990 Jan;420:149–163. doi: 10.1113/jphysiol.1990.sp017905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hiraoka M., Kawano S. Calcium-sensitive and insensitive transient outward current in rabbit ventricular myocytes. J Physiol. 1989 Mar;410:187–212. doi: 10.1113/jphysiol.1989.sp017528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume J. R. Comparative interactions of organic Ca++ channel antagonists with myocardial Ca++ and K+ channels. J Pharmacol Exp Ther. 1985 Jul;234(1):134–140. [PubMed] [Google Scholar]
- Imaizumi Y., Giles W. R. Quinidine-induced inhibition of transient outward current in cardiac muscle. Am J Physiol. 1987 Sep;253(3 Pt 2):H704–H708. doi: 10.1152/ajpheart.1987.253.3.H704. [DOI] [PubMed] [Google Scholar]
- Irisawa H., Kokubun S. Modulation by intracellular ATP and cyclic AMP of the slow inward current in isolated single ventricular cells of the guinea-pig. J Physiol. 1983 May;338:321–337. doi: 10.1113/jphysiol.1983.sp014675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson I. R., Sanchez-Chapula J., Brown A. M. Early outward current in rat single ventricular cells. Circ Res. 1984 Feb;54(2):157–162. doi: 10.1161/01.res.54.2.157. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Kakei M., Sato R., Shibasaki T., Matsuda H., Irisawa H. Intracellular Na+ activates a K+ channel in mammalian cardiac cells. Nature. 1984 May 24;309(5966):354–356. doi: 10.1038/309354a0. [DOI] [PubMed] [Google Scholar]
- Kass R. S. Delayed rectification in the cardiac Purkinje fiber is not activated by intracellular calcium. Biophys J. 1984 Apr;45(4):837–839. doi: 10.1016/S0006-3495(84)84227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass R. S., Tsien R. W. Multiple effects of calcium antagonists on plateau currents in cardiac Purkinje fibers. J Gen Physiol. 1975 Aug;66(2):169–192. doi: 10.1085/jgp.66.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature. 1983 Apr 28;302(5911):790–794. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Sugiyama K. A modulatory action of divalent cations on transient outward current in cultured rat sensory neurones. J Physiol. 1988 Feb;396:417–433. doi: 10.1113/jphysiol.1988.sp016970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. F., Pelzer D., Trautwein W. Cat ventricular muscle treated with D600: characteristics of calcium channel block and unblock. J Physiol. 1984 Jul;352:217–241. doi: 10.1113/jphysiol.1984.sp015288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. F., Pelzer D., Trautwein W. Cat ventricular muscle treated with D600: effects on calcium and potassium currents. J Physiol. 1984 Jul;352:203–216. doi: 10.1113/jphysiol.1984.sp015287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T., Pelzer D., Trautwein W. Dual action (stimulation, inhibition) of D600 on contractility and calcium channels in guinea-pig and cat heart cells. J Physiol. 1989 Jul;414:569–586. doi: 10.1113/jphysiol.1989.sp017704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R., Morad M. A uniform enzymatic method for dissociation of myocytes from hearts and stomachs of vertebrates. Am J Physiol. 1985 Nov;249(5 Pt 2):H1056–H1060. doi: 10.1152/ajpheart.1985.249.5.H1056. [DOI] [PubMed] [Google Scholar]
- Mubagwa K., Carmeliet E. Interaction of AOA 39, D600, verapamil and diltiazem with cardiac cholinergic effects. Arch Int Pharmacodyn Ther. 1987 Mar;286(1):71–84. [PubMed] [Google Scholar]
- Nakao M., Gadsby D. C. Voltage dependence of Na translocation by the Na/K pump. Nature. 1986 Oct 16;323(6089):628–630. doi: 10.1038/323628a0. [DOI] [PubMed] [Google Scholar]
- Nakayama T., Fozzard H. A. Adrenergic modulation of the transient outward current in isolated canine Purkinje cells. Circ Res. 1988 Jan;62(1):162–172. doi: 10.1161/01.res.62.1.162. [DOI] [PubMed] [Google Scholar]
- Nawrath H., Eick R. E., McDonald T. F., Trautwein W. On the mechanism underlying the action of D-600 on slow inward current and tension in mammalian myocardium. Circ Res. 1977 Apr;40(4):408–414. doi: 10.1161/01.res.40.4.408. [DOI] [PubMed] [Google Scholar]
- Payet M. D., Schanne O. F., Ruiz-Ceretti E., Demers J. M. Inhibitory activity of blockers of the slow inward current in rat myocardium, a study in steady state and of rate of action. J Mol Cell Cardiol. 1980 Feb;12(2):187–200. doi: 10.1016/0022-2828(80)90088-7. [DOI] [PubMed] [Google Scholar]
- Provencher S. W. A Fourier method for the analysis of exponential decay curves. Biophys J. 1976 Jan;16(1):27–41. doi: 10.1016/S0006-3495(76)85660-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatani A., Kunze D. L., Brown A. M. Effects of dihydropyridine calcium channel modulators on cardiac sodium channels. Am J Physiol. 1988 Jan;254(1 Pt 2):H140–H147. doi: 10.1152/ajpheart.1988.254.1.H140. [DOI] [PubMed] [Google Scholar]


