Abstract
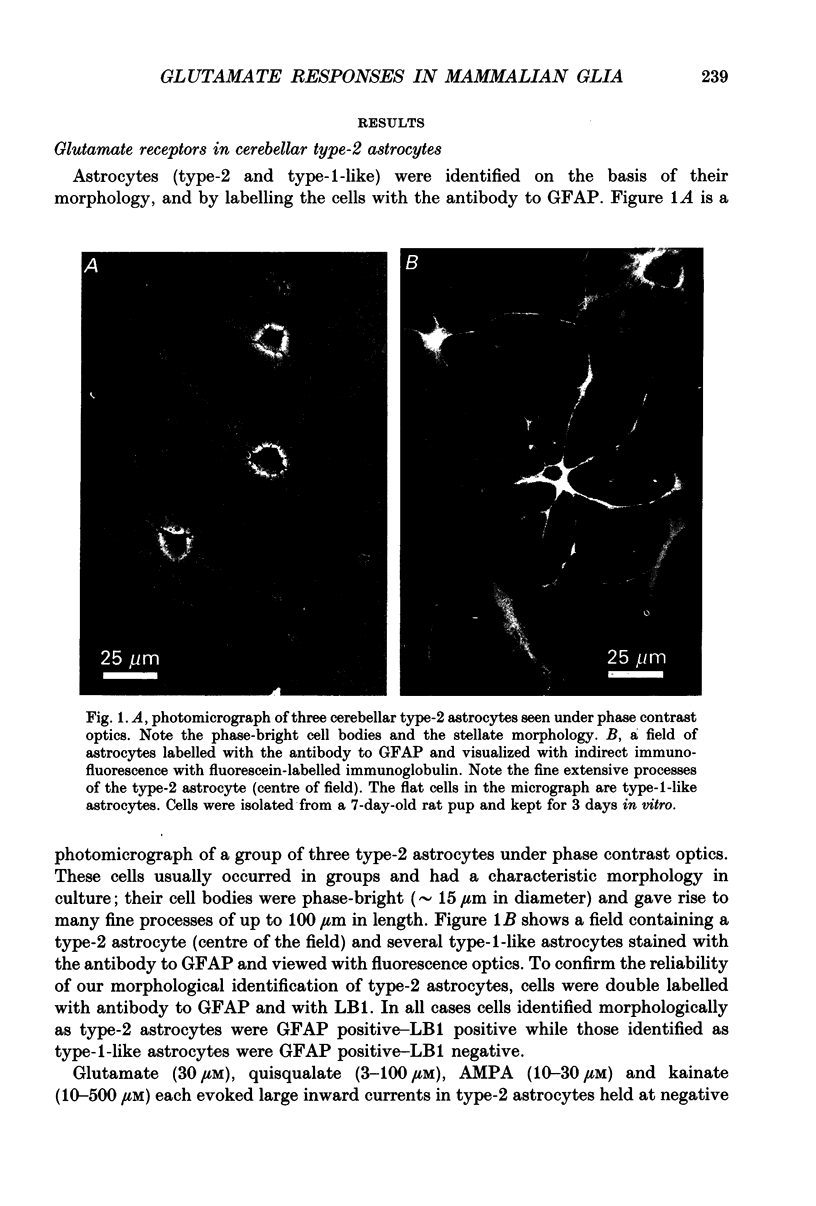
1. Patch-clamp methods have been used to examine the action of excitatory amino acids on three types of glial cell in cultures of rat cerebellum, namely type-1-like astrocytes, type-2 astrocytes and oligodendrocytes. In addition we have examined glutamate sensitivity of the precursor cell (the O-2A progenitor) that gives rise to type-2 astrocytes and oligodendrocytes. 2. Glutamate (30 microM), quisqualate (3-100 microM), (S)-alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA, 10-30 microM) and kainate (10-500 microM) were applied to cerebellar type-2 astrocytes examined under whole-cell voltage clamp. Each of these agonists induced inward currents in cells held at negative membrane potentials. The currents reversed direction near 0 mV holding potential. N-Methyl-D-aspartate (NMDA, 30-100 microM) or aspartate (30 microM) in the presence of glycine (1 microM) did not evoke any whole-cell current changes in type-2 astrocytes. 3. The distribution of glutamate receptors in type-2 astrocytes was mapped with single- or double-barrelled ionophoretic pipettes containing quisqualate or kainate. Application of these agonists (current pulses 100 ms, 50-100 nA) to cells held at -60 mV evoked inward currents of 20-120 pA in the cell soma and 10-80 pA in the processes. Responses could also be obtained at the extremities of processes (approximately 60 microns from the soma). 4. Quisqualate or kainate (at 30 microM) applied to O-2A progenitor cells from rat cerebellum or optic nerve induced whole-cell currents (quisqualate 20-30 pA; kainate 20-50 pA, holding potential, Vh = -60 mV) that reversed near 0 mV. In common with type-2 astrocytes, the progenitor cells did not respond to NMDA (30 microM). 5. Type-1-like astrocytes produced large inward currents to glutamate (30 microM). These currents remained inward-going at holding potentials as positive as +80 mV and were not accompanied by any apparent noise increase. This result can be explained by the presence of an electrogenic glutamate uptake carrier. In cells kept up to 4 days in vitro, quisqualate, kainate and NMDA each failed to produce any whole-cell current changes, indicating the absence of receptors in type-1-like astrocytes at this stage in culture. Furthermore the glutamate uptake currents in type-1-like astrocytes were inhibited when external Na+ was replaced by Li+, although Li+ was found to pass through the glutamate channel in type-2 astrocytes.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
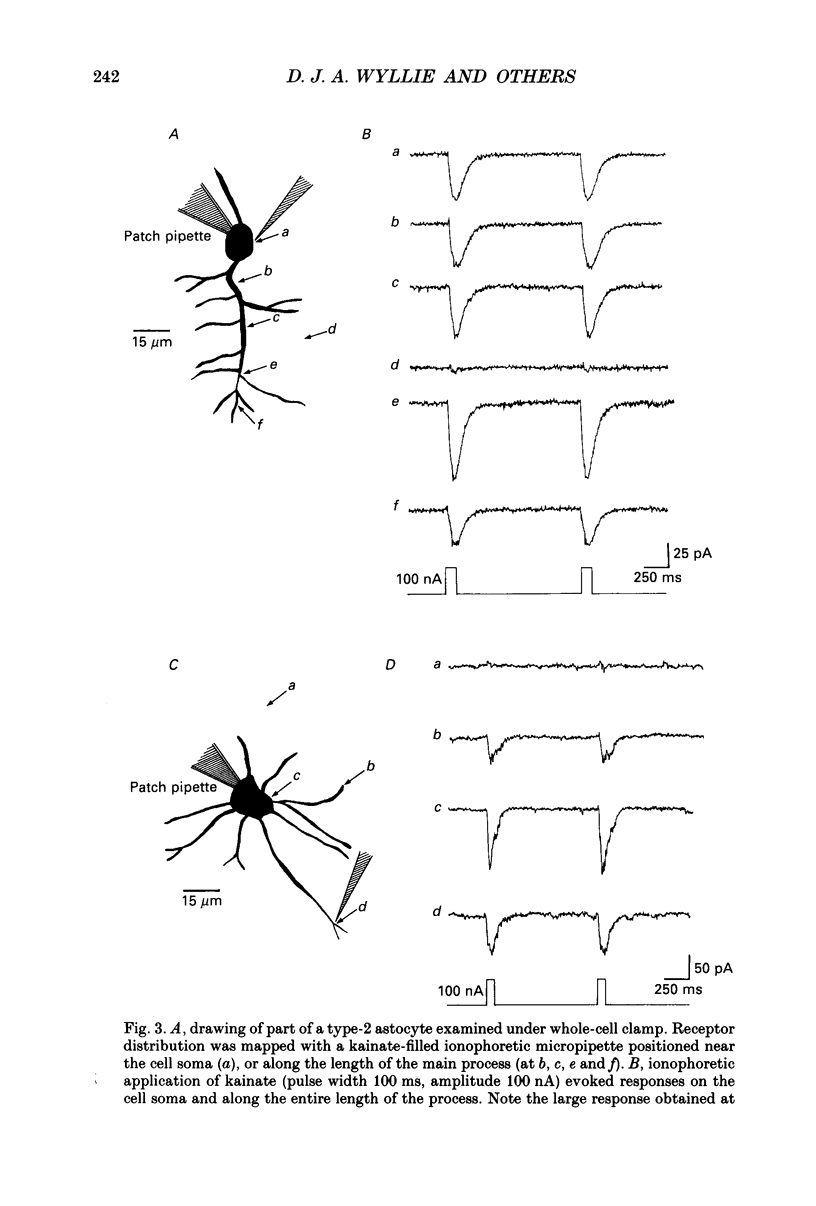
PDF




















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Kaneda M., Hori N., Krishtal O. A. Blockade of N-methyl-D-aspartate response in enzyme-treated rat hippocampal neurons. Neurosci Lett. 1988 Apr 22;87(1-2):75–79. doi: 10.1016/0304-3940(88)90148-6. [DOI] [PubMed] [Google Scholar]
- Allen C. N., Brady R., Swann J., Hori N., Carpenter D. O. N-methyl-D-aspartate (NMDA) receptors are inactivated by trypsin. Brain Res. 1988 Aug 16;458(1):147–150. doi: 10.1016/0006-8993(88)90507-0. [DOI] [PubMed] [Google Scholar]
- Antonov S. M., Magazanik L. G. Intense non-quantal release of glutamate in an insect neuromuscular junction. Neurosci Lett. 1988 Nov 11;93(2-3):204–208. doi: 10.1016/0304-3940(88)90082-1. [DOI] [PubMed] [Google Scholar]
- Ascher P., Nowak L. Quisqualate- and kainate-activated channels in mouse central neurones in culture. J Physiol. 1988 May;399:227–245. doi: 10.1113/jphysiol.1988.sp017077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backus K. H., Kettenmann H., Schachner M. Effect of benzodiazepines and pentobarbital on the GABA-induced depolarization in cultured astrocytes. Glia. 1988;1(2):132–140. doi: 10.1002/glia.440010205. [DOI] [PubMed] [Google Scholar]
- Balcar V. J., Johnston G. A. High affinity uptake of L-Glutamine in rat brain slices. J Neurochem. 1975 May;24(5):875–879. doi: 10.1111/j.1471-4159.1975.tb03650.x. [DOI] [PubMed] [Google Scholar]
- Balcar V. J., Johnston G. A. The structural specificity of the high affinity uptake of L-glutamate and L-aspartate by rat brain slices. J Neurochem. 1972 Nov;19(11):2657–2666. doi: 10.1111/j.1471-4159.1972.tb01325.x. [DOI] [PubMed] [Google Scholar]
- Balázs R., Jørgensen O. S., Hack N. N-methyl-D-aspartate promotes the survival of cerebellar granule cells in culture. Neuroscience. 1988 Nov;27(2):437–451. doi: 10.1016/0306-4522(88)90279-5. [DOI] [PubMed] [Google Scholar]
- Barbour B., Brew H., Attwell D. Electrogenic glutamate uptake in glial cells is activated by intracellular potassium. Nature. 1988 Sep 29;335(6189):433–435. doi: 10.1038/335433a0. [DOI] [PubMed] [Google Scholar]
- Barres B. A., Chun L. L., Corey D. P. Ion channel expression by white matter glia: I. Type 2 astrocytes and oligodendrocytes. Glia. 1988;1(1):10–30. doi: 10.1002/glia.440010104. [DOI] [PubMed] [Google Scholar]
- Barres B. A., Koroshetz W. J., Swartz K. J., Chun L. L., Corey D. P. Ion channel expression by white matter glia: the O-2A glial progenitor cell. Neuron. 1990 Apr;4(4):507–524. doi: 10.1016/0896-6273(90)90109-s. [DOI] [PubMed] [Google Scholar]
- Bignami A., Dahl D. Specificity of the glial fibrillary acidic protein for astroglia. J Histochem Cytochem. 1977 Jun;25(6):466–469. doi: 10.1177/25.6.69656. [DOI] [PubMed] [Google Scholar]
- Bormann J., Kettenmann H. Patch-clamp study of gamma-aminobutyric acid receptor Cl- channels in cultured astrocytes. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9336–9340. doi: 10.1073/pnas.85.23.9336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman C. L., Kimelberg H. K. Excitatory amino acids directly depolarize rat brain astrocytes in primary culture. Nature. 1984 Oct 18;311(5987):656–659. doi: 10.1038/311656a0. [DOI] [PubMed] [Google Scholar]
- Brew H., Attwell D. Electrogenic glutamate uptake is a major current carrier in the membrane of axolotl retinal glial cells. 1987 Jun 25-Jul 1Nature. 327(6124):707–709. doi: 10.1038/327707a0. [DOI] [PubMed] [Google Scholar]
- Cornell-Bell A. H., Finkbeiner S. M., Cooper M. S., Smith S. J. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990 Jan 26;247(4941):470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Howe J. R., Ogden D. C. Noise and single channels activated by excitatory amino acids in rat cerebellar granule neurones. J Physiol. 1988 Jun;400:189–222. doi: 10.1113/jphysiol.1988.sp017117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Ogden D. C. Ion channels activated by L-glutamate and GABA in cultured cerebellar neurons of the rat. Proc R Soc Lond B Biol Sci. 1985 May 22;224(1236):367–373. doi: 10.1098/rspb.1985.0038. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. Multiple-conductance channels activated by excitatory amino acids in cerebellar neurons. Nature. 1987 Feb 5;325(6104):525–528. doi: 10.1038/325525a0. [DOI] [PubMed] [Google Scholar]
- Curtis R., Cohen J., Fok-Seang J., Hanley M. R., Gregson N. A., Reynolds R., Wilkin G. P. Development of macroglial cells in rat cerebellum. I. Use of antibodies to follow early in vivo development and migration of oligodendrocytes. J Neurocytol. 1988 Feb;17(1):43–54. doi: 10.1007/BF01735376. [DOI] [PubMed] [Google Scholar]
- Desai M. A., Conn P. J. Selective activation of phosphoinositide hydrolysis by a rigid analogue of glutamate. Neurosci Lett. 1990 Feb 5;109(1-2):157–162. doi: 10.1016/0304-3940(90)90555-n. [DOI] [PubMed] [Google Scholar]
- Erecińska M. The neurotransmitter amino acid transport systems. A fresh outlook on an old problem. Biochem Pharmacol. 1987 Nov 1;36(21):3547–3555. doi: 10.1016/0006-2952(87)90001-3. [DOI] [PubMed] [Google Scholar]
- Evans P. D. The stability of the free amino acid pool in isolated peripheral nerves of Carcinus maenas (L.). J Exp Biol. 1973 Oct;59(2):463–476. doi: 10.1242/jeb.59.2.463. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C., Raff M. C. The oligodendrocyte-type-2 astrocyte cell lineage is specialized for myelination. 1986 Sep 25-Oct 1Nature. 323(6086):335–338. doi: 10.1038/323335a0. [DOI] [PubMed] [Google Scholar]
- Gallo V., Giovannini C., Suergiu R., Levi G. Expression of excitatory amino acid receptors by cerebellar cells of the type-2 astrocyte cell lineage. J Neurochem. 1989 Jan;52(1):1–9. doi: 10.1111/j.1471-4159.1989.tb10890.x. [DOI] [PubMed] [Google Scholar]
- Gregor P., Mano I., Maoz I., McKeown M., Teichberg V. I. Molecular structure of the chick cerebellar kainate-binding subunit of a putative glutamate receptor. Nature. 1989 Dec 7;342(6250):689–692. doi: 10.1038/342689a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hildebrand C. Ultrastructural and light-microscopic studies of the nodal region in large myelinated fibres of the adult feline spinal cord white matter. Acta Physiol Scand Suppl. 1971;364:43–79. doi: 10.1111/j.1365-201x.1971.tb10978.x. [DOI] [PubMed] [Google Scholar]
- Honoré T., Davies S. N., Drejer J., Fletcher E. J., Jacobsen P., Lodge D., Nielsen F. E. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988 Aug 5;241(4866):701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature. 1987 Feb 5;325(6104):522–525. doi: 10.1038/325522a0. [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Transmitter leakage from motor nerve endings. Proc R Soc Lond B Biol Sci. 1977 Feb 11;196(1122):59–72. doi: 10.1098/rspb.1977.0029. [DOI] [PubMed] [Google Scholar]
- Levi G., Gallo V., Ciotti M. T. Bipotential precursors of putative fibrous astrocytes and oligodendrocytes in rat cerebellar cultures express distinct surface features and "neuron-like" gamma-aminobutyric acid transport. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1504–1508. doi: 10.1073/pnas.83.5.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J. M., Stallcup W. B. Plasticity of developing cerebellar cells in vitro studied with antibodies against the NG2 antigen. J Neurosci. 1987 Sep;7(9):2721–2731. doi: 10.1523/JNEUROSCI.07-09-02721.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman E. M., Abbott N. J., Hassan S. Evidence that glutamate mediates axon-to-Schwann cell signaling in the squid. Glia. 1989;2(2):94–102. doi: 10.1002/glia.440020205. [DOI] [PubMed] [Google Scholar]
- Lillien L. E., Raff M. C. Analysis of the cell-cell interactions that control type-2 astrocyte development in vitro. Neuron. 1990 Apr;4(4):525–534. doi: 10.1016/0896-6273(90)90110-2. [DOI] [PubMed] [Google Scholar]
- MacVicar B. A., Baker K., Crichton S. A. Kainic acid evokes a potassium efflux from astrocytes. Neuroscience. 1988 May;25(2):721–725. doi: 10.1016/0306-4522(88)90272-2. [DOI] [PubMed] [Google Scholar]
- Marrero H., Astion M. L., Coles J. A., Orkand R. K. Facilitation of voltage-gated ion channels in frog neuroglia by nerve impulses. Nature. 1989 Jun 1;339(6223):378–380. doi: 10.1038/339378a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
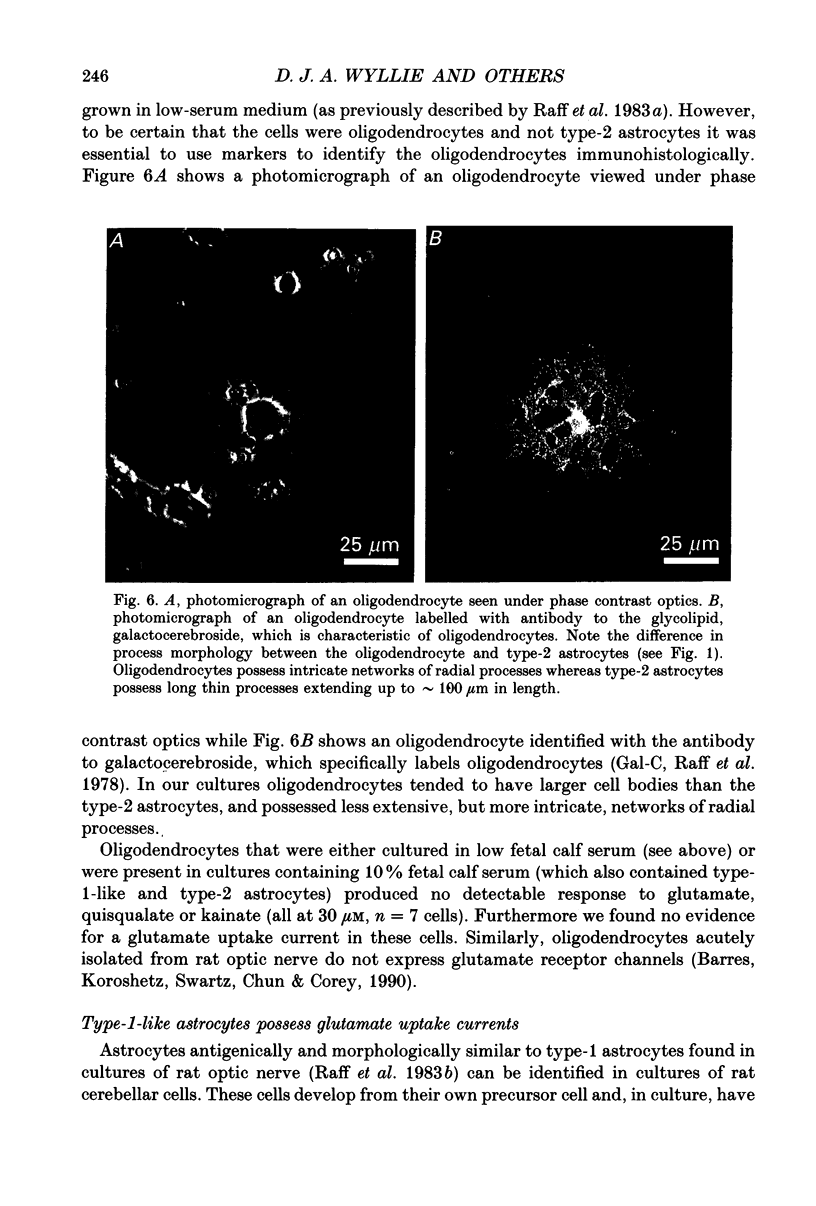
- Miller R. H., David S., Patel R., Abney E. R., Raff M. C. A quantitative immunohistochemical study of macroglial cell development in the rat optic nerve: in vivo evidence for two distinct astrocyte lineages. Dev Biol. 1985 Sep;111(1):35–41. doi: 10.1016/0012-1606(85)90432-4. [DOI] [PubMed] [Google Scholar]
- Miller R. H., Ffrench-Constant C., Raff M. C. The macroglial cells of the rat optic nerve. Annu Rev Neurosci. 1989;12:517–534. doi: 10.1146/annurev.ne.12.030189.002505. [DOI] [PubMed] [Google Scholar]
- Miller Robert H., Fulton Barbara P., Raff Martin C. A Novel Type of Glial Cell Associated with Nodes of Ranvier in Rat Optic Nerve. Eur J Neurosci. 1989 Mar;1(2):172–180. doi: 10.1111/j.1460-9568.1989.tb00785.x. [DOI] [PubMed] [Google Scholar]
- Murphy S., Pearce B. Functional receptors for neurotransmitters on astroglial cells. Neuroscience. 1987 Aug;22(2):381–394. doi: 10.1016/0306-4522(87)90342-3. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Patterson P. H. On the importance of being inhibited, or saying no to growth cones. Neuron. 1988 Jun;1(4):263–267. doi: 10.1016/0896-6273(88)90074-8. [DOI] [PubMed] [Google Scholar]
- Pearce I. A., Cambray-Deakin M. A., Burgoyne R. D. Glutamate acting on NMDA receptors stimulates neurite outgrowth from cerebellar granule cells. FEBS Lett. 1987 Oct 19;223(1):143–147. doi: 10.1016/0014-5793(87)80525-2. [DOI] [PubMed] [Google Scholar]
- Peterson N. A., Raghupathy E. Selective effects of lithium on synaptosomal amino acid transport systems. Biochem Pharmacol. 1974 Sep 1;23(17):2491–2494. doi: 10.1016/0006-2952(74)90249-4. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Abney E. R., Cohen J., Lindsay R., Noble M. Two types of astrocytes in cultures of developing rat white matter: differences in morphology, surface gangliosides, and growth characteristics. J Neurosci. 1983 Jun;3(6):1289–1300. doi: 10.1523/JNEUROSCI.03-06-01289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Abney E. R., Fok-Seang J. Reconstitution of a developmental clock in vitro: a critical role for astrocytes in the timing of oligodendrocyte differentiation. Cell. 1985 Aug;42(1):61–69. doi: 10.1016/s0092-8674(85)80101-x. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Fields K. L., Hakomori S. I., Mirsky R., Pruss R. M., Winter J. Cell-type-specific markers for distinguishing and studying neurons and the major classes of glial cells in culture. Brain Res. 1979 Oct 5;174(2):283–308. doi: 10.1016/0006-8993(79)90851-5. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Glial cell diversification in the rat optic nerve. Science. 1989 Mar 17;243(4897):1450–1455. doi: 10.1126/science.2648568. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Miller R. H., Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983 Jun 2;303(5916):390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Mirsky R., Fields K. L., Lisak R. P., Dorfman S. H., Silberberg D. H., Gregson N. A., Leibowitz S., Kennedy M. C. Galactocerebroside is a specific cell-surface antigenic marker for oligodendrocytes in culture. Nature. 1978 Aug 24;274(5673):813–816. [PubMed] [Google Scholar]
- Schwartz E. A. Depolarization without calcium can release gamma-aminobutyric acid from a retinal neuron. Science. 1987 Oct 16;238(4825):350–355. doi: 10.1126/science.2443977. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A., Tachibana M. Electrophysiology of glutamate and sodium co-transport in a glial cell of the salamander retina. J Physiol. 1990 Jul;426:43–80. doi: 10.1113/jphysiol.1990.sp018126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small R. K., Riddle P., Noble M. Evidence for migration of oligodendrocyte--type-2 astrocyte progenitor cells into the developing rat optic nerve. Nature. 1987 Jul 9;328(6126):155–157. doi: 10.1038/328155a0. [DOI] [PubMed] [Google Scholar]
- Somogyi P., Eshhar N., Teichberg V. I., Roberts J. D. Subcellular localization of a putative kainate receptor in Bergmann glial cells using a monoclonal antibody in the chick and fish cerebellar cortex. Neuroscience. 1990;35(1):9–30. doi: 10.1016/0306-4522(90)90116-l. [DOI] [PubMed] [Google Scholar]
- Sontheimer H., Kettenmann H., Backus K. H., Schachner M. Glutamate opens Na+/K+ channels in cultured astrocytes. Glia. 1988;1(5):328–336. doi: 10.1002/glia.440010505. [DOI] [PubMed] [Google Scholar]
- Sugiyama H., Ito I., Hirono C. A new type of glutamate receptor linked to inositol phospholipid metabolism. Nature. 1987 Feb 5;325(6104):531–533. doi: 10.1038/325531a0. [DOI] [PubMed] [Google Scholar]
- Traynelis S. F., Cull-Candy S. G. Proton inhibition of N-methyl-D-aspartate receptors in cerebellar neurons. Nature. 1990 May 24;345(6273):347–350. doi: 10.1038/345347a0. [DOI] [PubMed] [Google Scholar]
- Usowicz M. M., Gallo V., Cull-Candy S. G. Multiple conductance channels in type-2 cerebellar astrocytes activated by excitatory amino acids. Nature. 1989 Jun 1;339(6223):380–383. doi: 10.1038/339380a0. [DOI] [PubMed] [Google Scholar]
- Vyskocil F., Nikolsky E., Edwards C. An analysis of the mechanisms underlying the non-quantal release of acetylcholine at the mouse neuromuscular junction. Neuroscience. 1983 Jun;9(2):429–435. doi: 10.1016/0306-4522(83)90305-6. [DOI] [PubMed] [Google Scholar]
- Weinreich D., Hammerschlag R. Nerve impulse-enhanced release of amino acids from non-synaptic regions of peripheral and central nerve trunks of bullfrog. Brain Res. 1975 Jan 24;84(1):137–142. doi: 10.1016/0006-8993(75)90807-0. [DOI] [PubMed] [Google Scholar]
- Wheeler D. D., Boyarsky L. L., Brooks W. H. The release of amino acids from nerve during stimulation. J Cell Physiol. 1966 Feb;67(1):141–147. doi: 10.1002/jcp.1040670116. [DOI] [PubMed] [Google Scholar]
- Wilkin G. P., Levi G., Johnstone S. R., Riddle P. N. Cerebellar astroglial cells in primary culture: expression of different morphological appearances and different ability to take up [3H]D-aspartate and [3H]GABA. Brain Res. 1983 Nov;312(2):265–277. doi: 10.1016/0165-3806(83)90143-8. [DOI] [PubMed] [Google Scholar]
- Wood J. N., Anderton B. H. Monoclonal antibodies to mammalian neurofilaments. Biosci Rep. 1981 Mar;1(3):263–268. doi: 10.1007/BF01114913. [DOI] [PubMed] [Google Scholar]





