Abstract
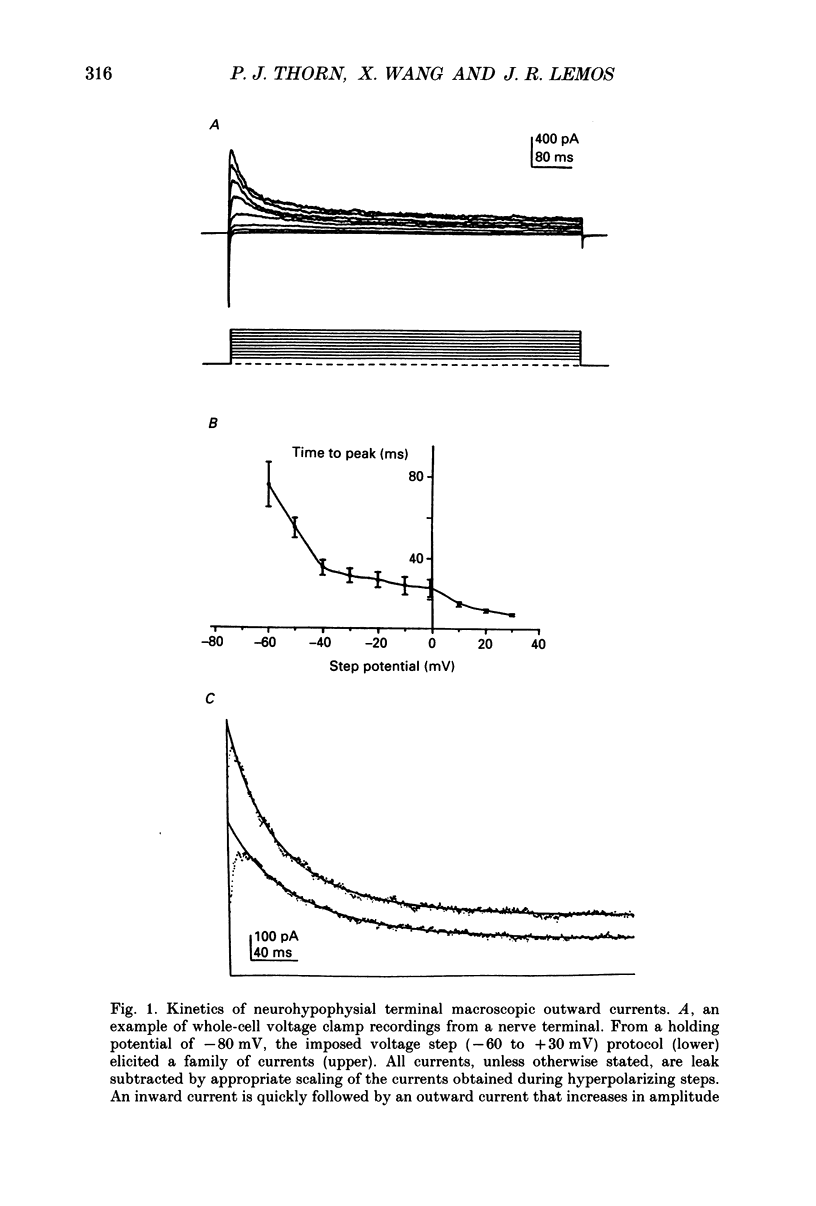
1. Nerve terminals of the rat posterior pituitary were acutely dissociated and identified using a combination of morphological and immunohistochemical techniques. Macroscopic terminal membrane currents and voltages were studied using the whole-cell patch clamp technique. 2. In physiological solutions, depolarizing voltage clamp steps, from a holding potential (-80 mV) similar to the normal terminal resting potential, elicited a fast, inward followed by a fast, transient, outward current. 3. The threshold of activation for the outward current was -60 mV. The outward current quickly reached a peak and then decayed more slowly. The decay was fitted by two exponentials with time constants of 21 +/- 2.9 and 143 +/- 36 ms. These decay constants did not show a dependence on voltage. The time to peak of the outward current decreased and the amplitude increased with increasingly depolarized potential steps. 4. The outward current was blocked by the substitution of K+ with Cs+ and its reversal potential was consistent with a potassium current. 5. The transient outward current showed steady-state inactivation at more depolarized (than -80 mV) holding potentials with 50% inactivation occurring at -47.9 mV. The time course of recovery from inactivation was complex with full recovery taking greater than 16 s. 6. 4-Aminopyridine (4-AP) blocked the transient outward current in a dose-dependent manner (approximately IC50 = 3 mM), while charybdotoxin (4 micrograms/ml) and tetraethylammonium (100 mM) had no effect on the current amplitude. 7. Lowering external [Ca2+] had no effect on the fast, transient outward current nor did the calcium channel blocker Cd2+ (2 mM). 8. The neurohypophysial outward current reported here corresponds most closely to IA, and not to the delayed rectifier or Ca2(+)-activated K+ currents. Neurohypophysial IA, however, appears to be different from the outward currents found in the cell bodies in the hypothalamus which project their axons to the posterior pituitary. 9. Under current clamp, evoked action potential duration increased (122%) upon application of 5 mM-4-AP, indicating that IA is involved in neurohypophysial spike repolarization. 10. The existence of this current could help explain why maximal peptide release only occurs in response to bursts of electrical activity invading the nerve terminals.
Full text
PDF





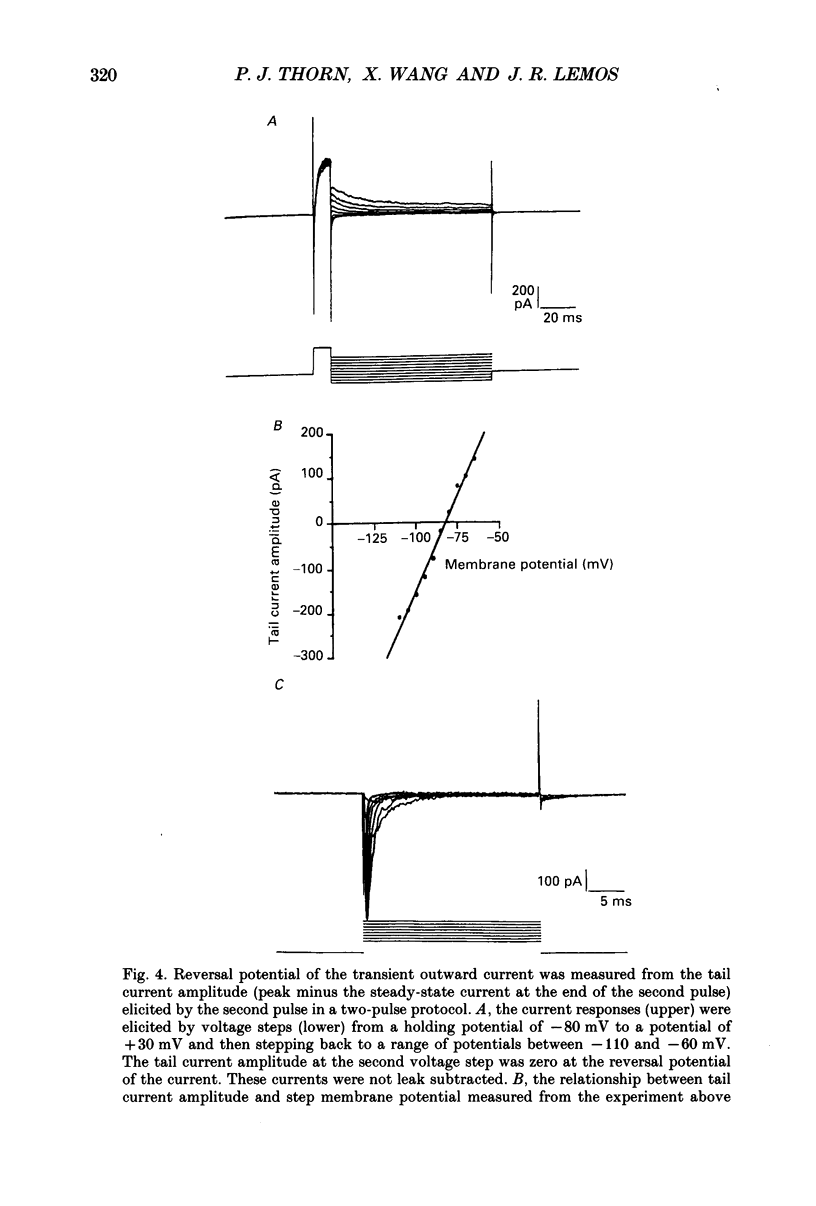





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
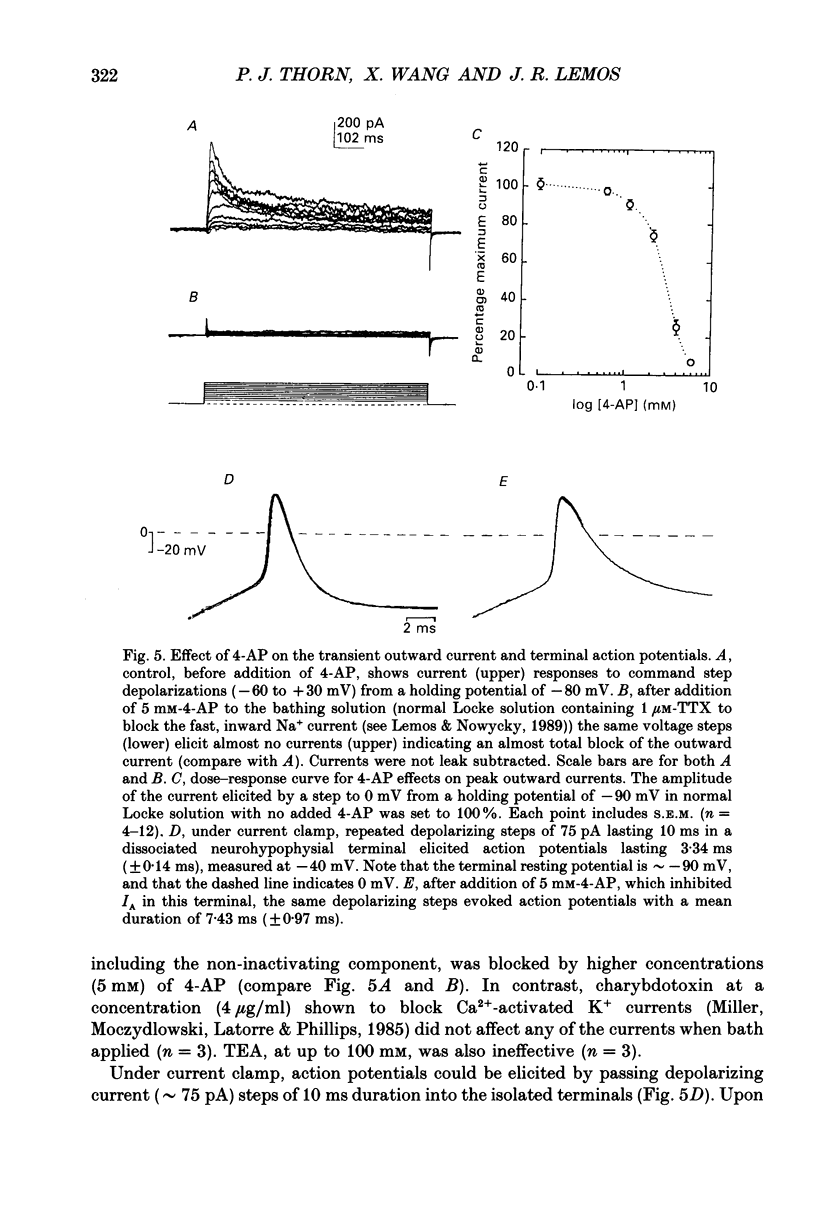
- Adams P. R., Brown D. A., Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982 Sep;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P., Smith S. J. Calcium action in synaptic transmitter release. Annu Rev Neurosci. 1987;10:633–693. doi: 10.1146/annurev.ne.10.030187.003221. [DOI] [PubMed] [Google Scholar]
- Belluzzi O., Sacchi O., Wanke E. A fast transient outward current in the rat sympathetic neurone studied under voltage-clamp conditions. J Physiol. 1985 Jan;358:91–108. doi: 10.1113/jphysiol.1985.sp015542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy C. A., Gainer H., Russell J. T. Effects of stimulus frequency and potassium channel blockade on the secretion of vasopressin and oxytocin from the neurohypophysis. Neuroendocrinology. 1987 Sep;46(3):258–267. doi: 10.1159/000124829. [DOI] [PubMed] [Google Scholar]
- Bourque C. W. Intraterminal recordings from the rat neurohypophysis in vitro. J Physiol. 1990 Feb;421:247–262. doi: 10.1113/jphysiol.1990.sp017943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque C. W. Transient calcium-dependent potassium current in magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol. 1988 Mar;397:331–347. doi: 10.1113/jphysiol.1988.sp017004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brethes D., Dayanithi G., Letellier L., Nordmann J. J. Depolarization-induced Ca2+ increase in isolated neurosecretory nerve terminals measured with fura-2. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1439–1443. doi: 10.1073/pnas.84.5.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalis M., Dayanithi G., Nordmann J. J. Hormone release from isolated nerve endings of the rat neurohypophysis. J Physiol. 1987 Sep;390:55–70. doi: 10.1113/jphysiol.1987.sp016686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalis M., Dayanithi G., Nordmann J. J. The role of patterned burst and interburst interval on the excitation-coupling mechanism in the isolated rat neural lobe. J Physiol. 1985 Dec;369:45–60. doi: 10.1113/jphysiol.1985.sp015887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Inward and delayed outward membrane currents in isolated neural somata under voltage clamp. J Physiol. 1971 Feb;213(1):1–19. doi: 10.1113/jphysiol.1971.sp009364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper E., Shrier A. Single-channel analysis of fast transient potassium currents from rat nodose neurones. J Physiol. 1985 Dec;369:199–208. doi: 10.1113/jphysiol.1985.sp015896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan M., Sedlmeir C. Outward currents in voltage-clamped rat sympathetic neurones. J Physiol. 1984 Nov;356:115–133. doi: 10.1113/jphysiol.1984.sp015456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B., Galvan M., Grafe P., Wigström H. A transient outward current in a mammalian central neurone blocked by 4-aminopyridine. Nature. 1982 Sep 16;299(5880):252–254. doi: 10.1038/299252a0. [DOI] [PubMed] [Google Scholar]
- HAGIWARA S., KUSANO K., SAITO N. Membrane changes of Onchidium nerve cell in potassium-rich media. J Physiol. 1961 Mar;155:470–489. doi: 10.1113/jphysiol.1961.sp006640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Lemos J. R., Nordmann J. J., Cooke I. M., Stuenkel E. L. Single channels and ionic currents in peptidergic nerve terminals. 1986 Jan 30-Feb 5Nature. 319(6052):410–412. doi: 10.1038/319410a0. [DOI] [PubMed] [Google Scholar]
- Lemos J. R., Nordmann J. J. Ionic channels and hormone release from peptidergic nerve terminals. J Exp Biol. 1986 Sep;124:53–72. doi: 10.1242/jeb.124.1.53. [DOI] [PubMed] [Google Scholar]
- Lemos J. R., Nowycky M. C. Two types of calcium channels coexist in peptide-releasing vertebrate nerve terminals. Neuron. 1989 May;2(5):1419–1426. doi: 10.1016/0896-6273(89)90187-6. [DOI] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Presynaptic calcium currents in squid giant synapse. Biophys J. 1981 Mar;33(3):289–321. doi: 10.1016/S0006-3495(81)84898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Sugiyama K. A modulatory action of divalent cations on transient outward current in cultured rat sensory neurones. J Physiol. 1988 Feb;396:417–433. doi: 10.1113/jphysiol.1988.sp016970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C., Moczydlowski E., Latorre R., Phillips M. Charybdotoxin, a protein inhibitor of single Ca2+-activated K+ channels from mammalian skeletal muscle. Nature. 1985 Jan 24;313(6000):316–318. doi: 10.1038/313316a0. [DOI] [PubMed] [Google Scholar]
- Neher E. Two fast transient current components during voltage clamp on snail neurons. J Gen Physiol. 1971 Jul;58(1):36–53. doi: 10.1085/jgp.58.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann J. J., Cazalis M., Dayanithi G., Castanas E., Giraud P., Legros J. J., Louis F. Are opioid peptides co-localized with vasopressin or oxytocin in the neural lobe of the rat? Cell Tissue Res. 1986;246(1):177–182. doi: 10.1007/BF00219015. [DOI] [PubMed] [Google Scholar]
- Nordmann J. J., Dayanithi G., Cazalis M. Do opioid peptides modulate, at the level of the nerve endings, the release of neurohypophysial hormones? Exp Brain Res. 1986;61(3):560–566. doi: 10.1007/BF00237581. [DOI] [PubMed] [Google Scholar]
- Nordmann J. J., Dayanithi G., Lemos J. R. Isolated neurosecretory nerve endings as a tool for studying the mechanism of stimulus-secretion coupling. Biosci Rep. 1987 May;7(5):411–426. doi: 10.1007/BF01362504. [DOI] [PubMed] [Google Scholar]
- Nordmann J. J., Desmazes J. P., Georgescauld D. The relationship between the membrane potential of neurosecretory nerve endings, as measured by a voltage-sensitive dye, and the release of neurohypophysial hormones. Neuroscience. 1982 Mar;7(3):731–737. doi: 10.1016/0306-4522(82)90078-1. [DOI] [PubMed] [Google Scholar]
- Nordmann J. J., Stuenkel E. L. Electrical properties of axons and neurohypophysial nerve terminals and their relationship to secretion in the rat. J Physiol. 1986 Nov;380:521–539. doi: 10.1113/jphysiol.1986.sp016300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain D. A., Wakerley J. B. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982 Apr;7(4):773–808. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Salzberg B. M., Obaid A. L. Optical studies of the secretory event at vertebrate nerve terminals. J Exp Biol. 1988 Sep;139:195–231. doi: 10.1242/jeb.139.1.195. [DOI] [PubMed] [Google Scholar]
- Salzberg B. M., Obaid A. L., Senseman D. M., Gainer H. Optical recording of action potentials from vertebrate nerve terminals using potentiometric probes provides evidence for sodium and calcium components. Nature. 1983 Nov 3;306(5938):36–40. doi: 10.1038/306036a0. [DOI] [PubMed] [Google Scholar]
- Shaw F. D., Bicknell R. J., Dyball R. E. Facilitation of vasopressin release from the neurohypophysis by application of electrical stimuli in bursts. Relevant stimulation parameters. Neuroendocrinology. 1984 Oct;39(4):371–376. doi: 10.1159/000124007. [DOI] [PubMed] [Google Scholar]
- Shimahara T. Presynaptic modulation of transmitter release by the early outward potassium current in Aplysia. Brain Res. 1983 Mar 14;263(1):51–56. doi: 10.1016/0006-8993(83)91199-x. [DOI] [PubMed] [Google Scholar]
- Zbicz K. L., Weight F. F. Transient voltage and calcium-dependent outward currents in hippocampal CA3 pyramidal neurons. J Neurophysiol. 1985 Apr;53(4):1038–1058. doi: 10.1152/jn.1985.53.4.1038. [DOI] [PubMed] [Google Scholar]


