Abstract
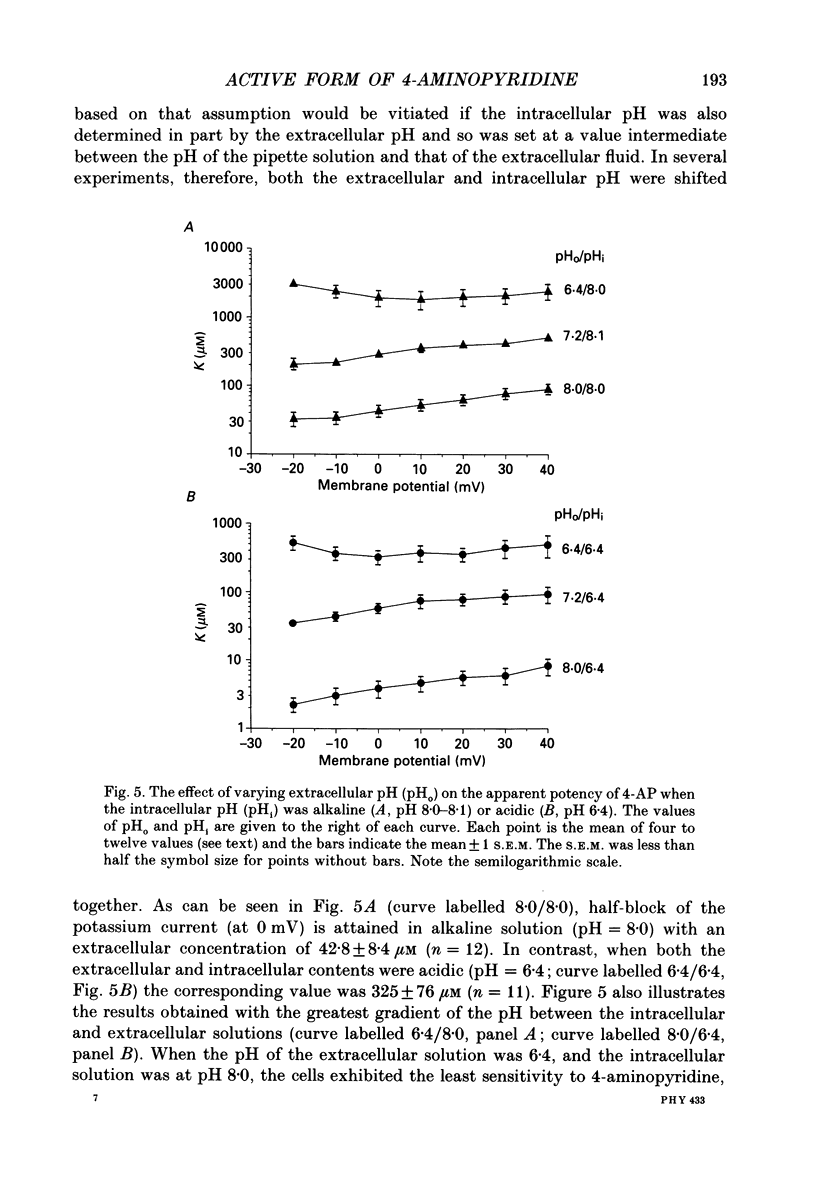
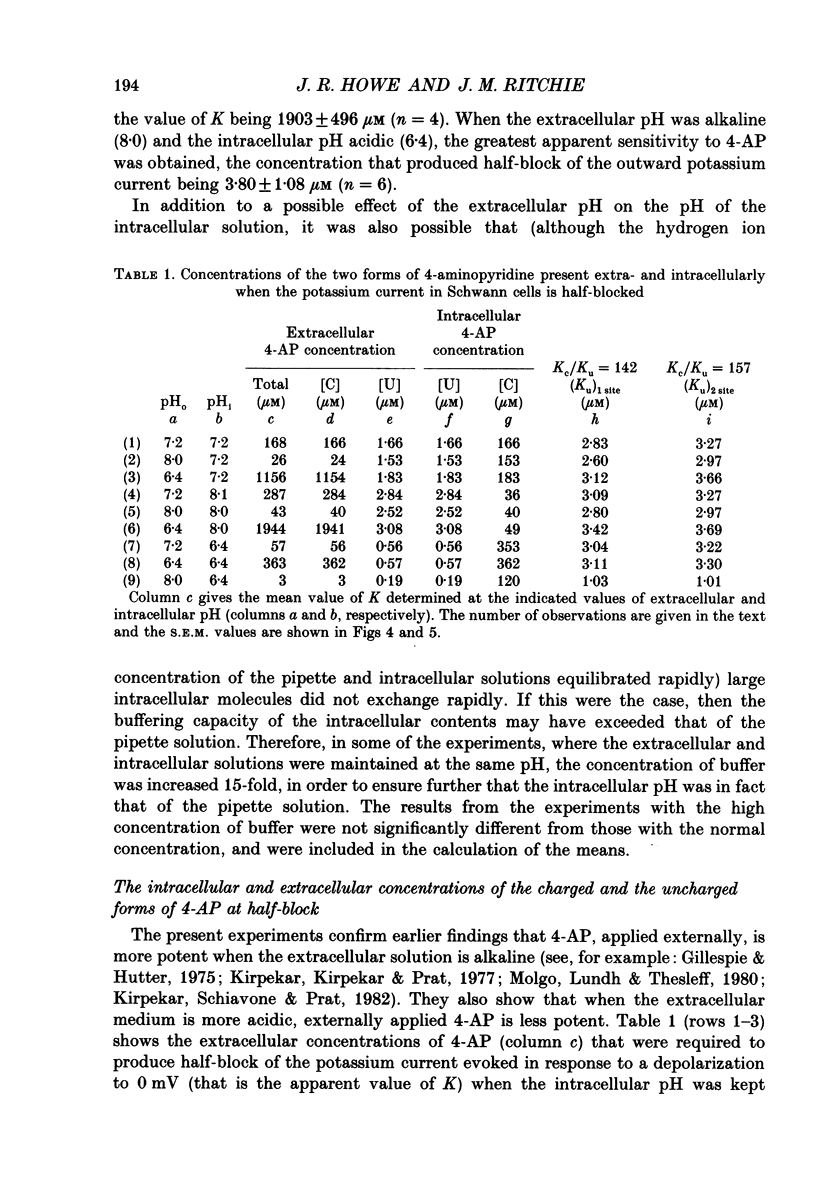
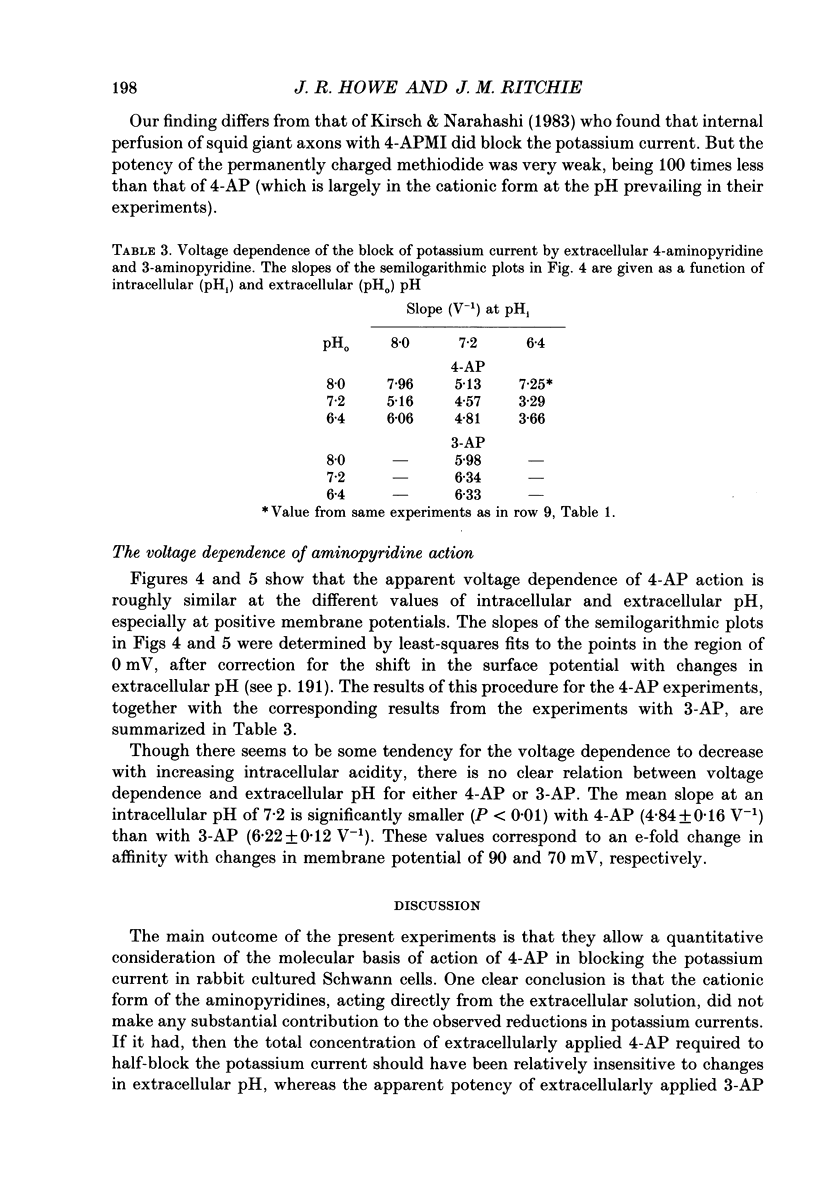
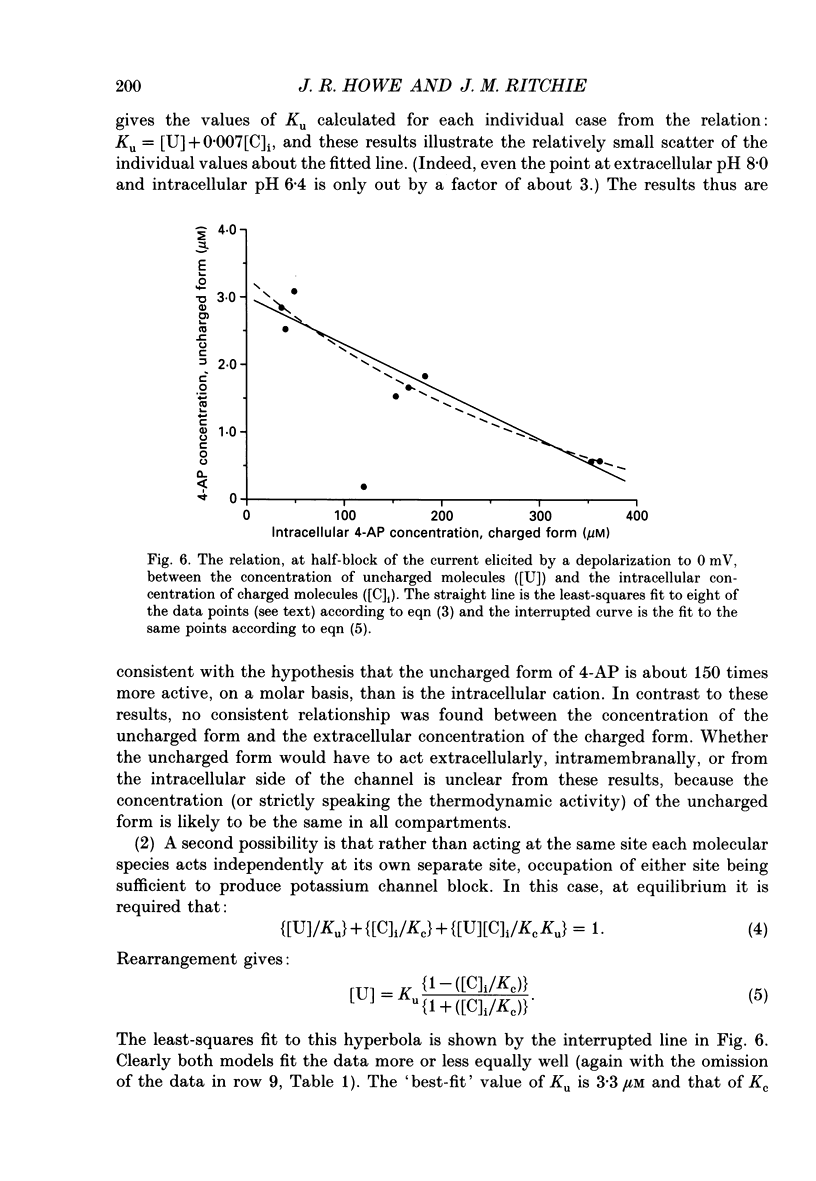
1. The blocking action of 4-aminopyridine (4-AP) on the outward potassium currents evoked by depolarization of rabbit Schwann cells in short-term primary culture was studied with the whole-cell configuration of the patch-clamp method. 2. We have determined the apparent equilibrium dissociation constant, K, for the action of 4-AP to block potassium currents at a series of different extracellular and intracellular pH values. 4-Aminopyridine is an organic base and exists in both charged and uncharged forms in aqueous solution. Changes in the pH of the extracellular and intracellular solutions therefore also change the extracellular and intracellular proportions of these two forms, and the values of K that were obtained were found to depend in a consistent way on both the extracellular and the intracellular pH. 3. At alkaline extracellular pH, K was decreased. At acidic extracellular pH, K was increased. In contrast, increasing the intracellular pH from 7.2 to 8.1 reduced the apparent potency of extracellularly applied 4-AP (i.e. increased K), and decreasing the intracellular pH (to 6.4) increased this apparent potency (i.e. decreased K). 4. The 4-AP analogues, 2-aminopyridine, 3-aminopyridine and 3,4-diaminopyridine, were also tested. At half-block of the potassium current, the intracellular concentration of the cationic form of the various aminopyridines (applied extracellularly at pH 7.2) varied by a factor of less than five, whereas that of the uncharged form varied by a factor of over 700. 5. The results are inconsistent with the hypothesis that the cationic form of the aminopyridines, acting from the extracellular solution, contributes in any substantial way to potassium channel block. It also seems unlikely that the uncharged form, acting either extracellularly or intracellularly, is solely responsible for the block. However, the results as a whole are consistent with the idea that it is the cation acting from the intracellular side that blocks the 4-AP-sensitive potassium channel and that the affinity with which 4-AP blocks the channel depends on the intracellular pH. The results would be explained if the cation competes with protons for a binding site that has an apparent pKa of about 7.0. 6. The results, nevertheless, are not inconsistent with the possibility that both the uncharged form and the intracellular charged form of 4-AP are active in blocking the 4-AP-sensitive potassium channel.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



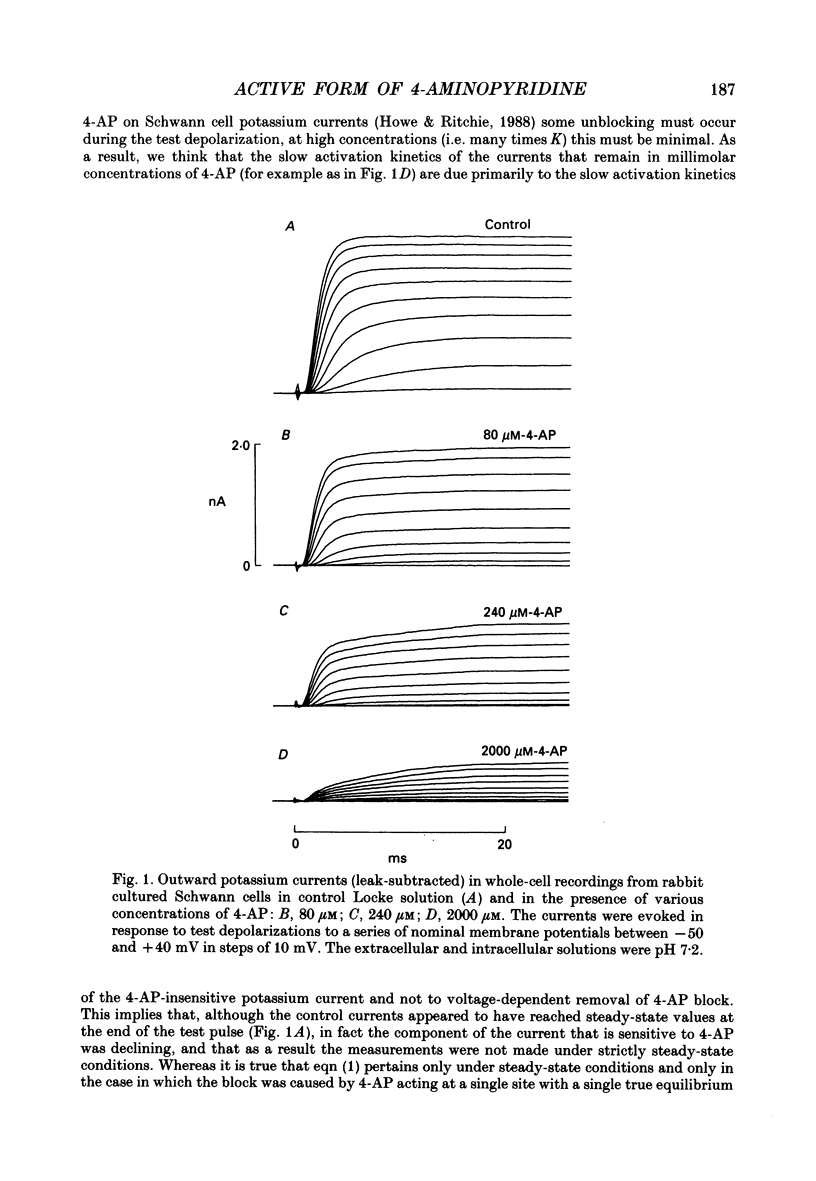
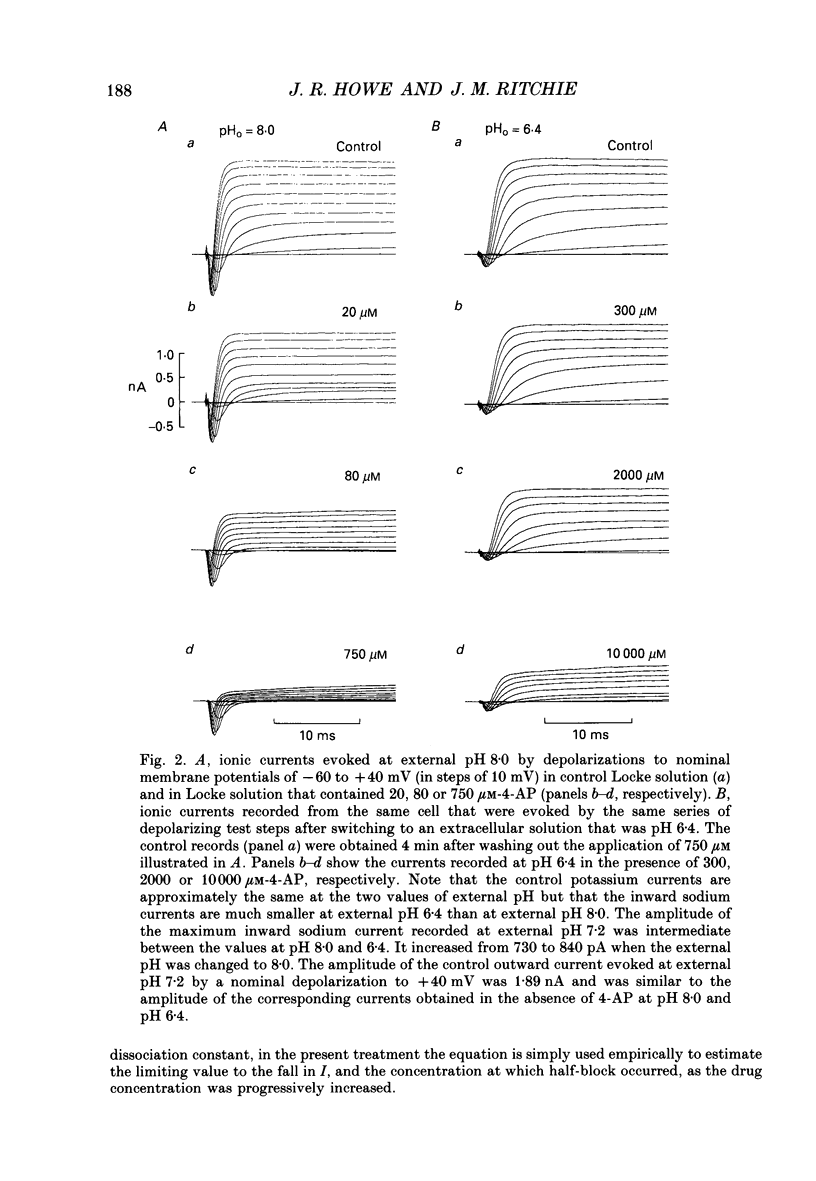

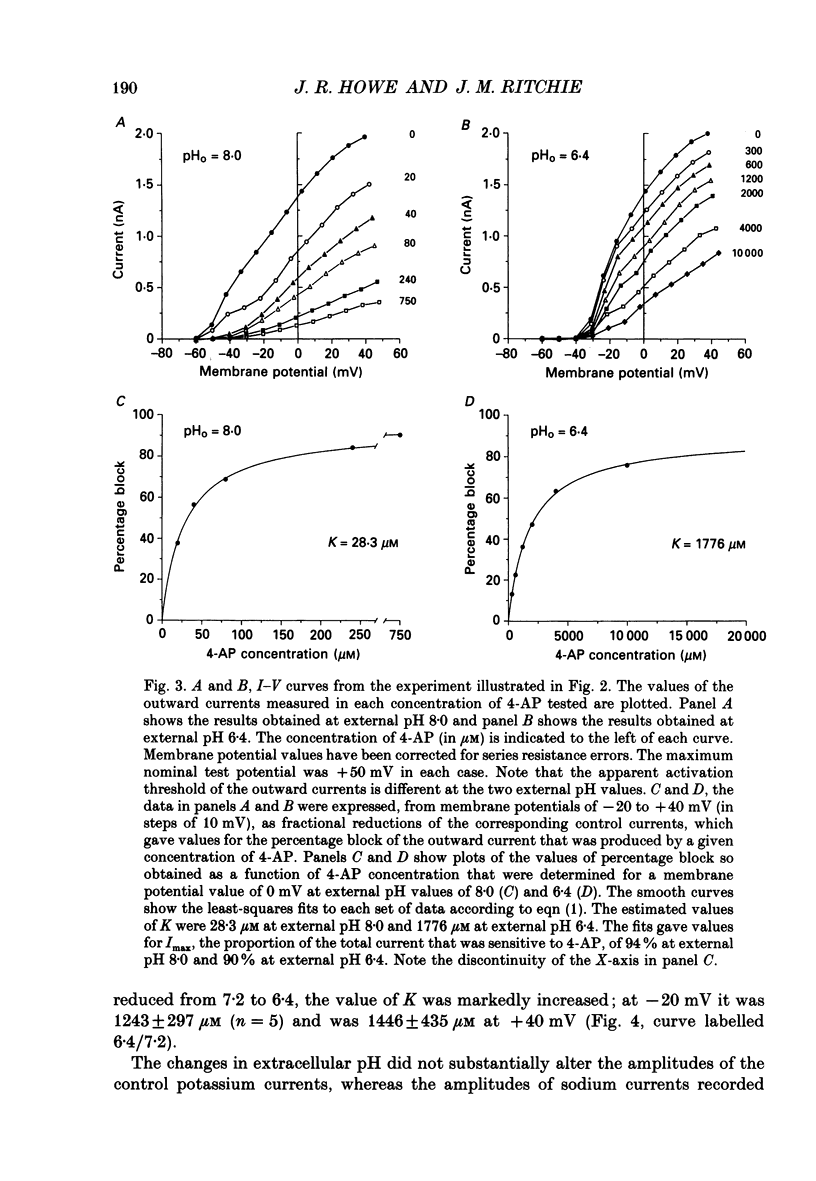

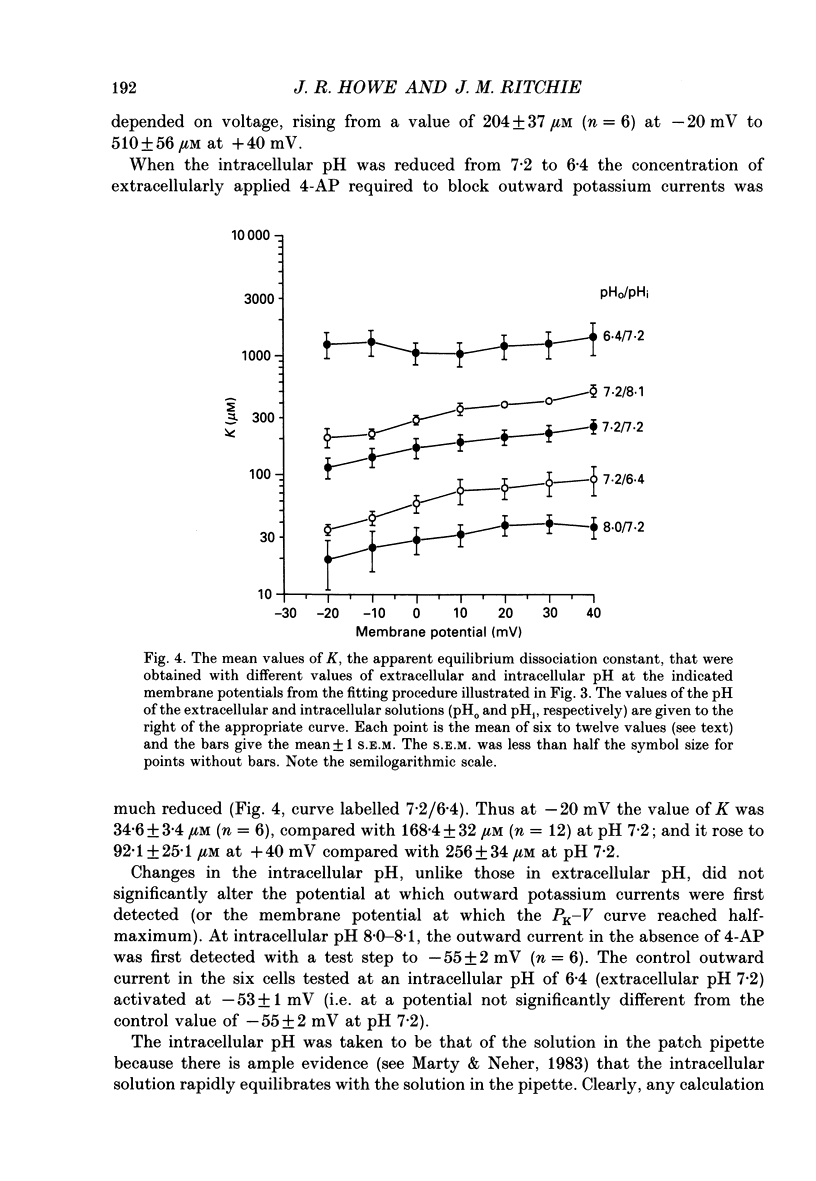









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blinks J. R., Wier W. G., Hess P., Prendergast F. G. Measurement of Ca2+ concentrations in living cells. Prog Biophys Mol Biol. 1982;40(1-2):1–114. doi: 10.1016/0079-6107(82)90011-6. [DOI] [PubMed] [Google Scholar]
- Chiu S. Y., Schrager P., Ritchie J. M. Neuronal-type Na+ and K+ channels in rabbit cultured Schwann cells. Nature. 1984 Sep 13;311(5982):156–157. doi: 10.1038/311156a0. [DOI] [PubMed] [Google Scholar]
- Gillespie J. I., Hutter O. F. Proceedings: The actions of 4-aminopyridine on the delayed potassium current in skeletal muscle fibres. J Physiol. 1975 Nov;252(2):70P–71P. [PubMed] [Google Scholar]
- Gray P. T., Ritchie J. M. A voltage-gated chloride conductance in rat cultured astrocytes. Proc R Soc Lond B Biol Sci. 1986 Aug 22;228(1252):267–288. doi: 10.1098/rspb.1986.0055. [DOI] [PubMed] [Google Scholar]
- Hille B. Charges and potentials at the nerve surface. Divalent ions and pH. J Gen Physiol. 1968 Feb;51(2):221–236. doi: 10.1085/jgp.51.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977 Apr;69(4):497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Potassium channels in myelinated nerve. Selective permeability to small cations. J Gen Physiol. 1973 Jun;61(6):669–686. doi: 10.1085/jgp.61.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn A. S., Lambert J. J., Marshall I. G. A comparison of the facilitatory actions of 4-aminopyridine methiodide and 4-aminopyridine on neuromuscular transmission. Br J Pharmacol. 1979 Jan;65(1):53–62. doi: 10.1111/j.1476-5381.1979.tb17333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. R., Ritchie J. M. Sodium currents in Schwann cells from myelinated and non-myelinated nerves of neonatal and adult rabbits. J Physiol. 1990 Jun;425:169–210. doi: 10.1113/jphysiol.1990.sp018098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. R., Ritchie J. M. Two types of potassium current in rabbit cultured Schwann cells. Proc R Soc Lond B Biol Sci. 1988 Oct 22;235(1278):19–27. doi: 10.1098/rspb.1988.0061. [DOI] [PubMed] [Google Scholar]
- Kirpekar M., Kirpekar S. M., Prat J. C. Effect of 4-aminopyridine on release of noradrenaline from the perfused cat spleen by nerve stimulation. J Physiol. 1977 Nov;272(3):517–528. doi: 10.1113/jphysiol.1977.sp012057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch G. E., Narahashi T. 3,4-diaminopyridine. A potent new potassium channel blocker. Biophys J. 1978 Jun;22(3):507–512. doi: 10.1016/S0006-3495(78)85503-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch G. E., Narahashi T. Site of action and active form of aminopyridines in squid axon membranes. J Pharmacol Exp Ther. 1983 Jul;226(1):174–179. [PubMed] [Google Scholar]
- Meves H., Pichon Y. The effect of internal and external 4-aminopyridine on the potassium currents in intracellularly perfused squid giant axons. J Physiol. 1977 Jun;268(2):511–532. doi: 10.1113/jphysiol.1977.sp011869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molgó J., Lundh H., Thesleff S. Potency of 3,4-diaminopyridine and 4-aminopyridine on mammalian neuromuscular transmission and the effect of pH changes. Eur J Pharmacol. 1980 Jan 11;61(1):25–34. doi: 10.1016/0014-2999(80)90378-7. [DOI] [PubMed] [Google Scholar]
- Mozhayeva G. N., Naumov A. P. Tetraethylammonium ion inhibition of potassium conductance of the nodal membrane. Biochim Biophys Acta. 1972 Dec 1;290(1):248–255. doi: 10.1016/0005-2736(72)90067-3. [DOI] [PubMed] [Google Scholar]
- Pelhate M., Pichon Y. Proceedings: Selective inhibition of potassium current in the giant axon of the cockroach. J Physiol. 1974 Oct;242(2):90P–91P. [PubMed] [Google Scholar]
- Shrager P., Chiu S. Y., Ritchie J. M. Voltage-dependent sodium and potassium channels in mammalian cultured Schwann cells. Proc Natl Acad Sci U S A. 1985 Feb;82(3):948–952. doi: 10.1073/pnas.82.3.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strichartz G. R. The inhibition of sodium currents in myelinated nerve by quaternary derivatives of lidocaine. J Gen Physiol. 1973 Jul;62(1):37–57. doi: 10.1085/jgp.62.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbricht W., Wagner H. H. Block of potassium channels of the nodal membrane by 4-aminopyridine and its partial removal on depolarization. Pflugers Arch. 1976 Nov 30;367(1):77–87. doi: 10.1007/BF00583659. [DOI] [PubMed] [Google Scholar]
- Wanke E., Carbone E., Testa P. L. K+ conductance modified by a titratable group accessible to protons from the intracellular side of the squid axon membrane. Biophys J. 1979 May;26(2):319–324. doi: 10.1016/S0006-3495(79)85251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J. Z., Oxford G. S., Wu C. H., Narahashi T. Dynamics of aminopyridine block of potassium channels in squid axon membrane. J Gen Physiol. 1976 Nov;68(5):519–535. doi: 10.1085/jgp.68.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J. Z., Oxford G. S., Wu C. H., Narahashi T. Interactions of aminopyridines with potassium channels of squid axon membranes. Biophys J. 1976 Jan;16(1):77–81. doi: 10.1016/S0006-3495(76)85663-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


