Abstract
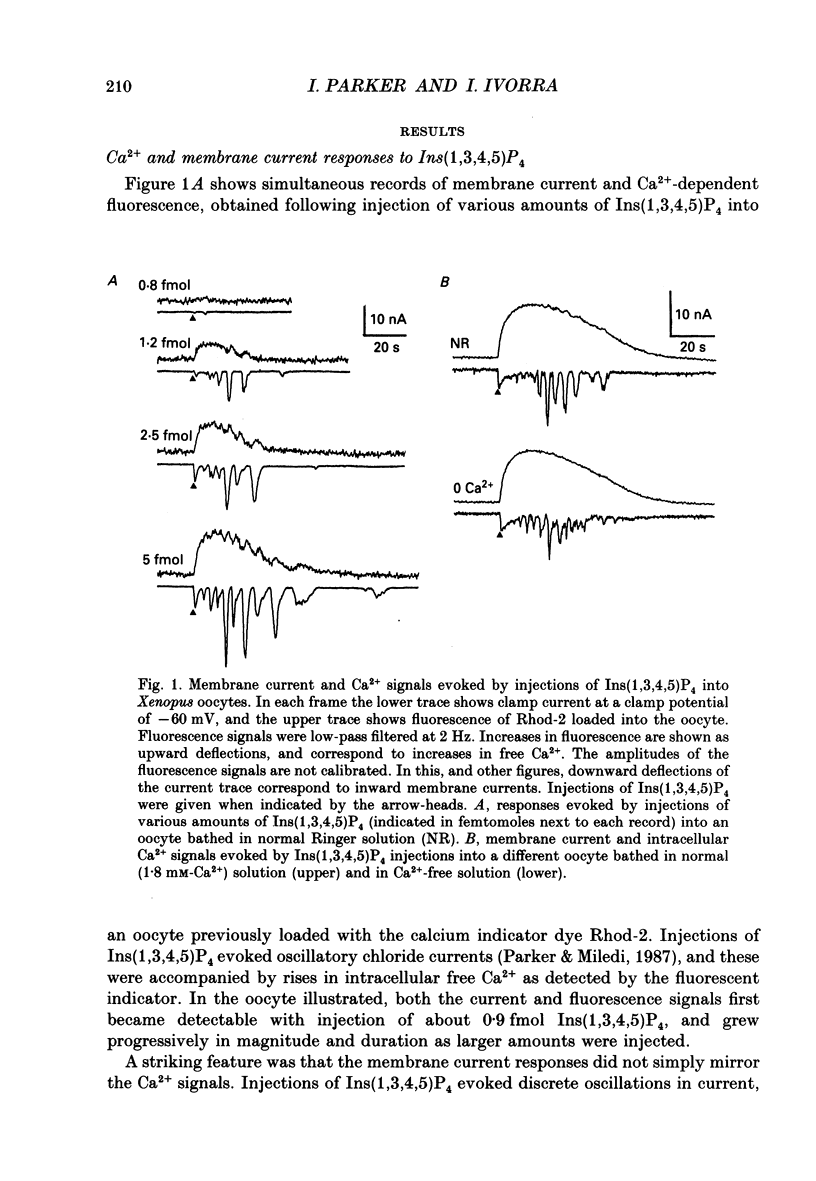
1. The actions of the putative second messenger inositol 1,3,4,5-tetrakisphosphate (Ins(1,3,4,5)P4) were studied by injecting it into voltage-clamped oocytes while recording Ca(2+)-dependent chloride membrane currents and, in some experiments, fluorescence signals from Ca2+ indicators. 2. Ins(1,3,4,5)P4 evoked a rise in intracellular Ca2+ and associated chloride current in oocytes bathed in normal or Ca(2+)-free Ringer solutions. The fluorescence Ca2+ signal showed a prolonged rise with superimposed oscillations, whereas the current reflected only the oscillatory component. 3. Injections of inositol 1,4,5-trisphosphate (Ins(1,4,5)P3) evoked currents showing an initial transient, followed by oscillations. Ins(1,3,4,5)P4 evoked similar oscillations, but the transient component was usually small or absent. Ins(1,3,4,5)P4 was about 20-fold less potent than Ins(1,4,5)P3, as measured by comparing doses required to elicit currents with the same integral. The most sensitive oocytes responded to about 1 fmol Ins(1,3,4,5)P4 and 0.1 fmol Ins(1,4,5)P3. 4. Injections of Ins(2,4,5)P4 evoked oscillatory currents, with a potency about three times greater than Ins(1,4,5)P3. Ins(1,3,4)P4 was ineffective in some oocytes even at doses of several picomoles, but in other oocytes evoked small transient and oscillatory currents with a potency 100 times or more less than Ins(1,3,4,5)P4. 5. Injections of Ins(1,3,4,5)P4 made into the animal hemisphere of the oocyte evoked larger currents than injections into the vegetal hemisphere. 6. Photo-release of Ins(1,4,5)P3 from caged Ins(1,4,5)P4 loaded into the oocyte was used to examine interactions between Ins(1,4,5)P3 and Ins(1,3,4,5)P4. Injection of low (ca 1 fmol) doses of Ins(1,3,4,5)P4 shortly before a light flash greatly facilitated currents evoked by photo-release of near-threshold amounts of Ins(1,4,5)P3. This facilitation was unaffected by removal of extracellular Ca2+ and arose because Ins(1,3,4,5)P4 reduced the threshold amount of Ins(1,4,5)P3 required to evoke a response. 7. Larger amounts (several femtomoles) of Ins(1,3,4,5)P4 depressed responses evoked by photo-release of Ins(1,4,5)P3. This may arise because Ca2+ liberated by Ins(1,3,4,5)P4 inhibits the ability of Ins(1,4,5)P3 to release further Ca2+. 8. We conclude that Ins(1,3,4,5)P4 liberates intracellular Ca2+ in the oocyte in a manner similar to that of Ins(1,4,5)P3, and suggest that a physiological role for Ins(1,3,4,5)P4 may be to facilitate responses to Ins(1,4,5)P3.
Full text
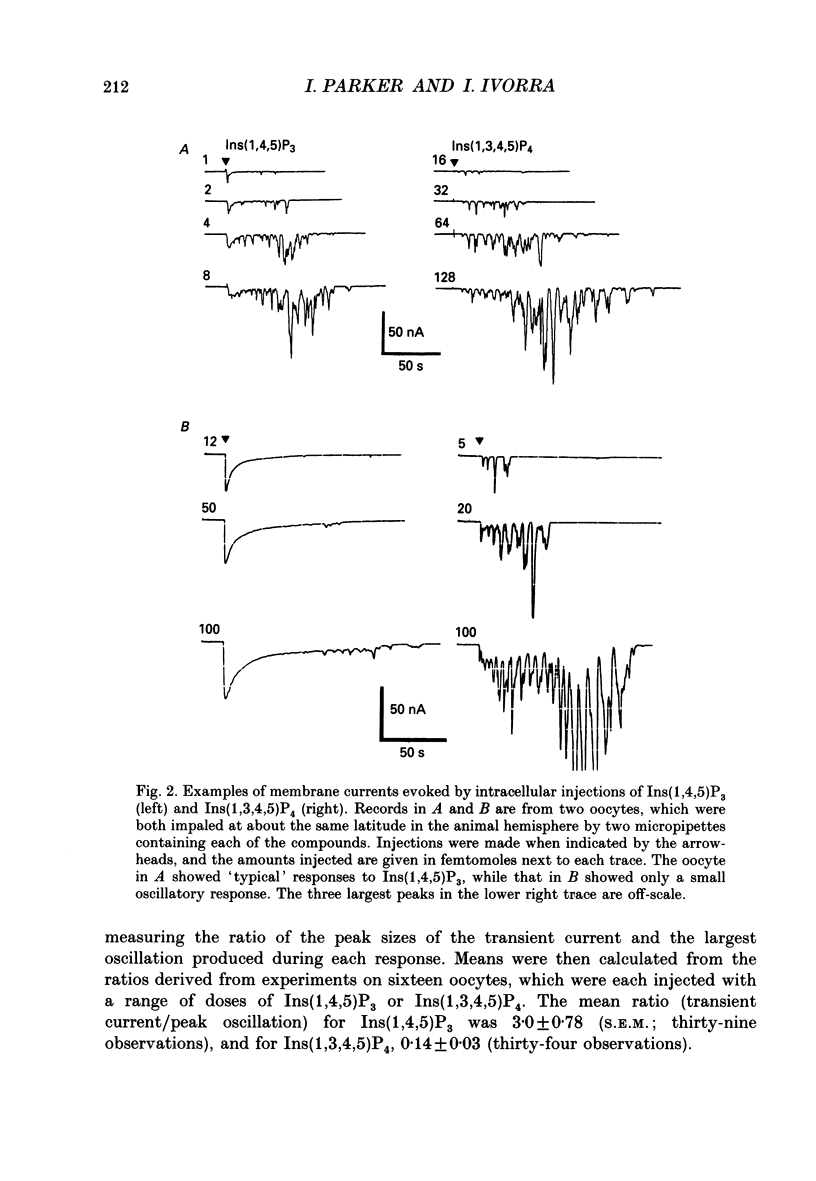
PDF



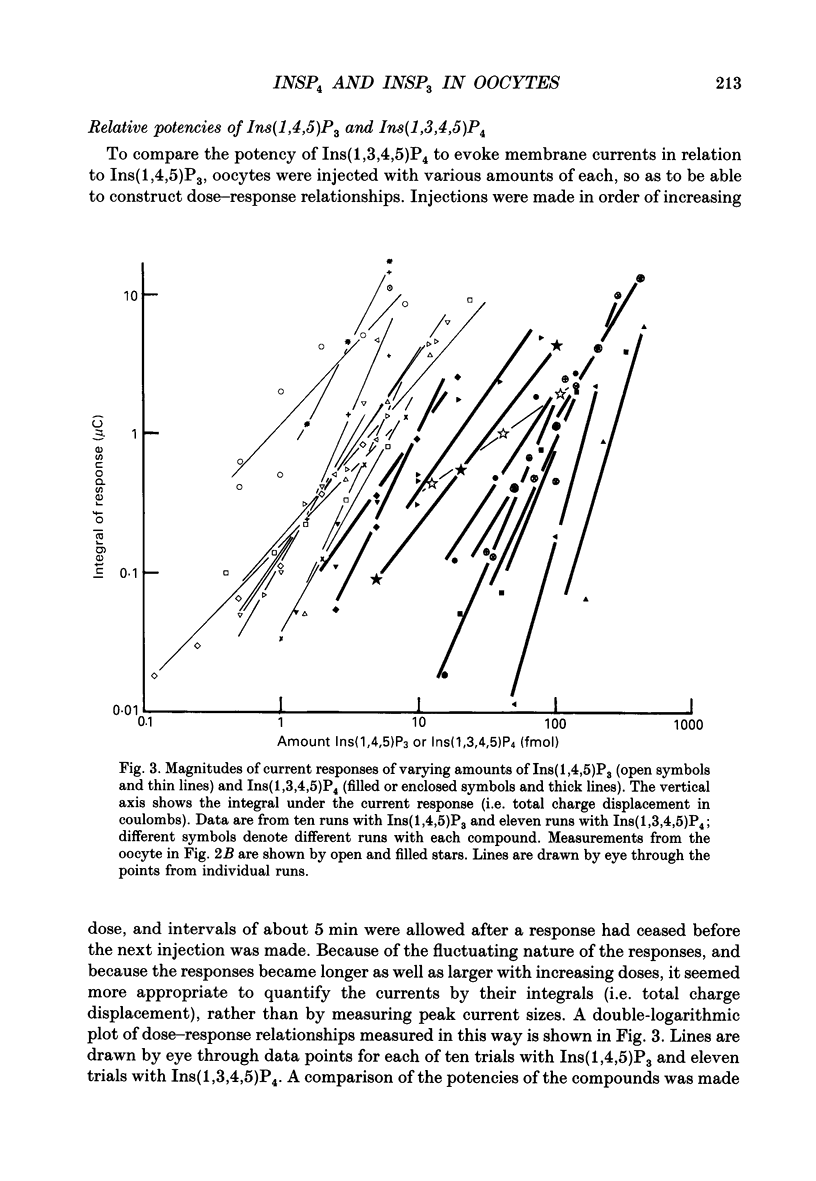
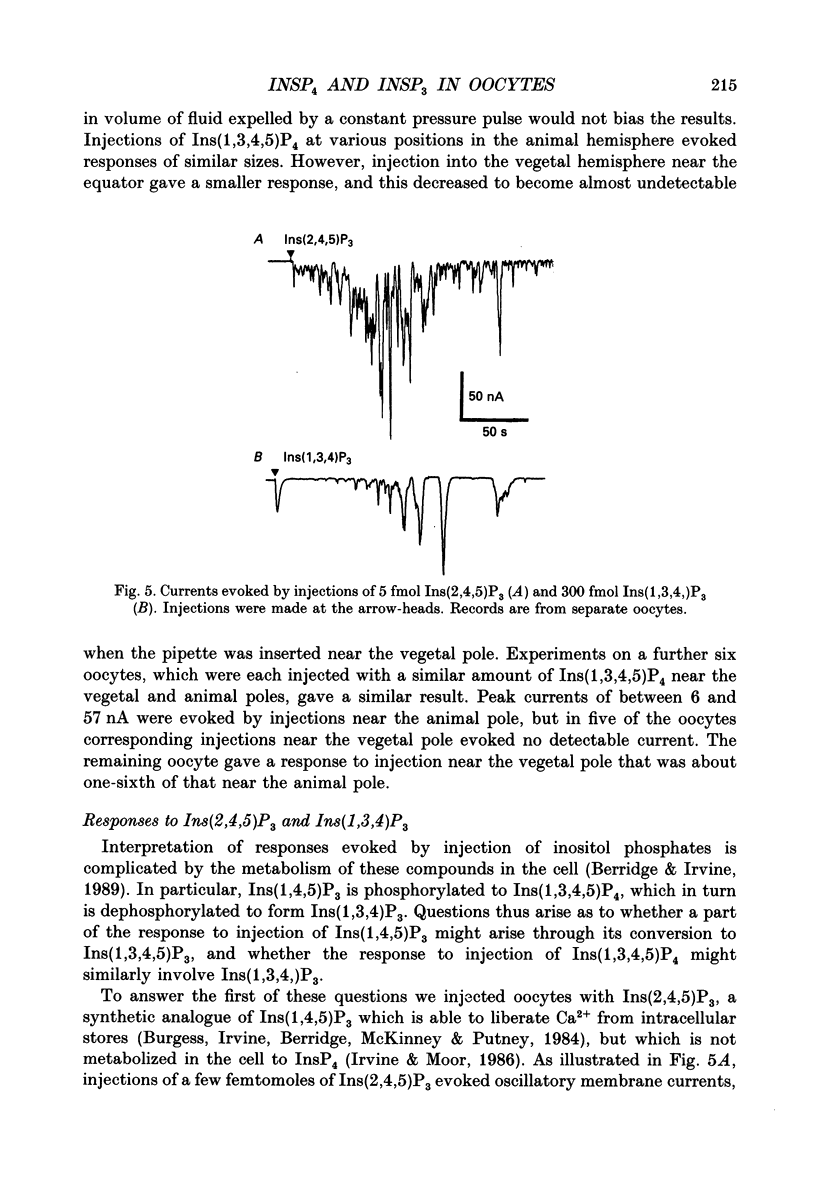
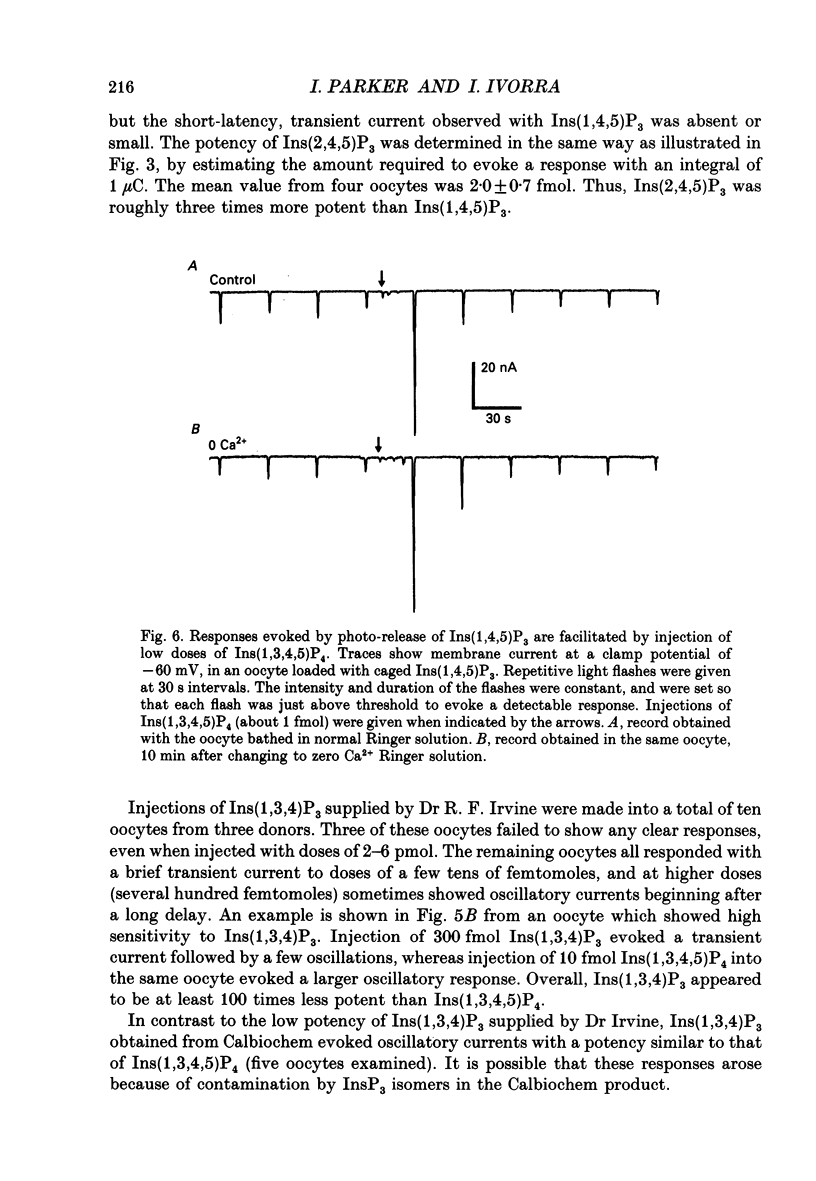

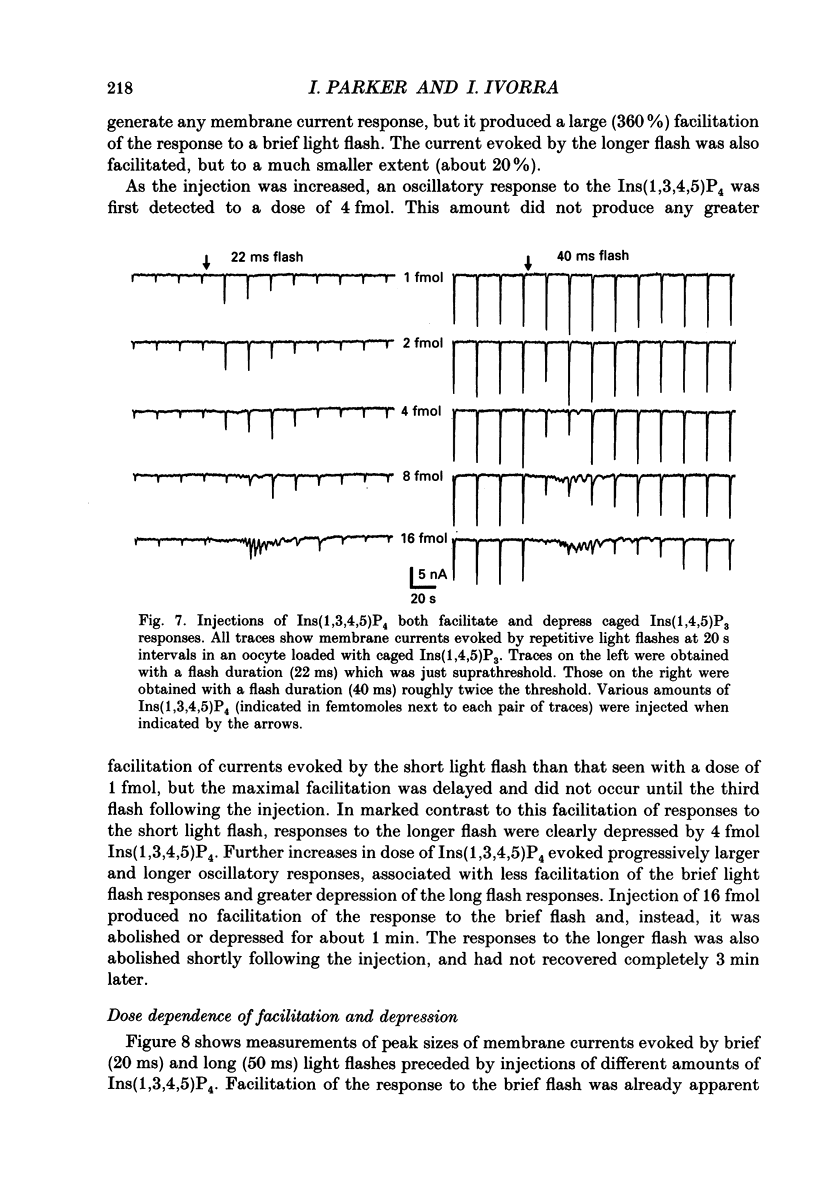
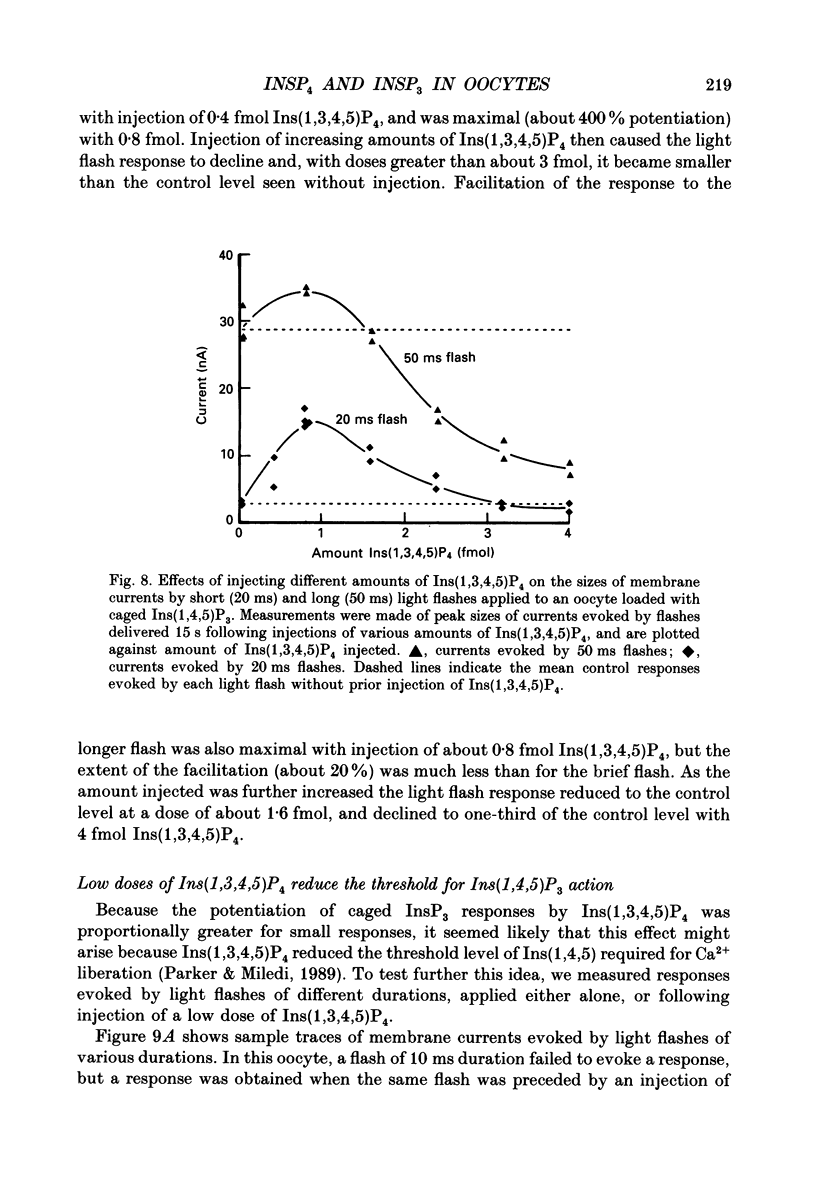







Selected References
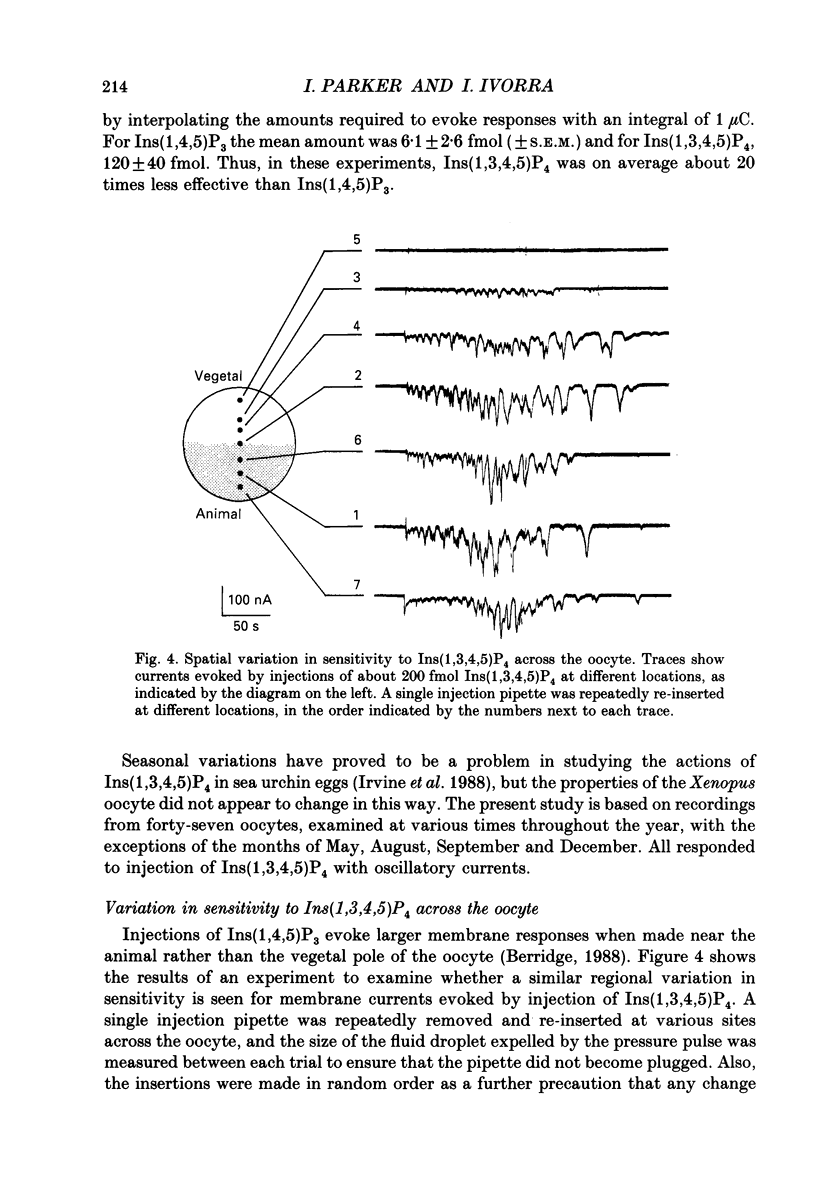
These references are in PubMed. This may not be the complete list of references from this article.
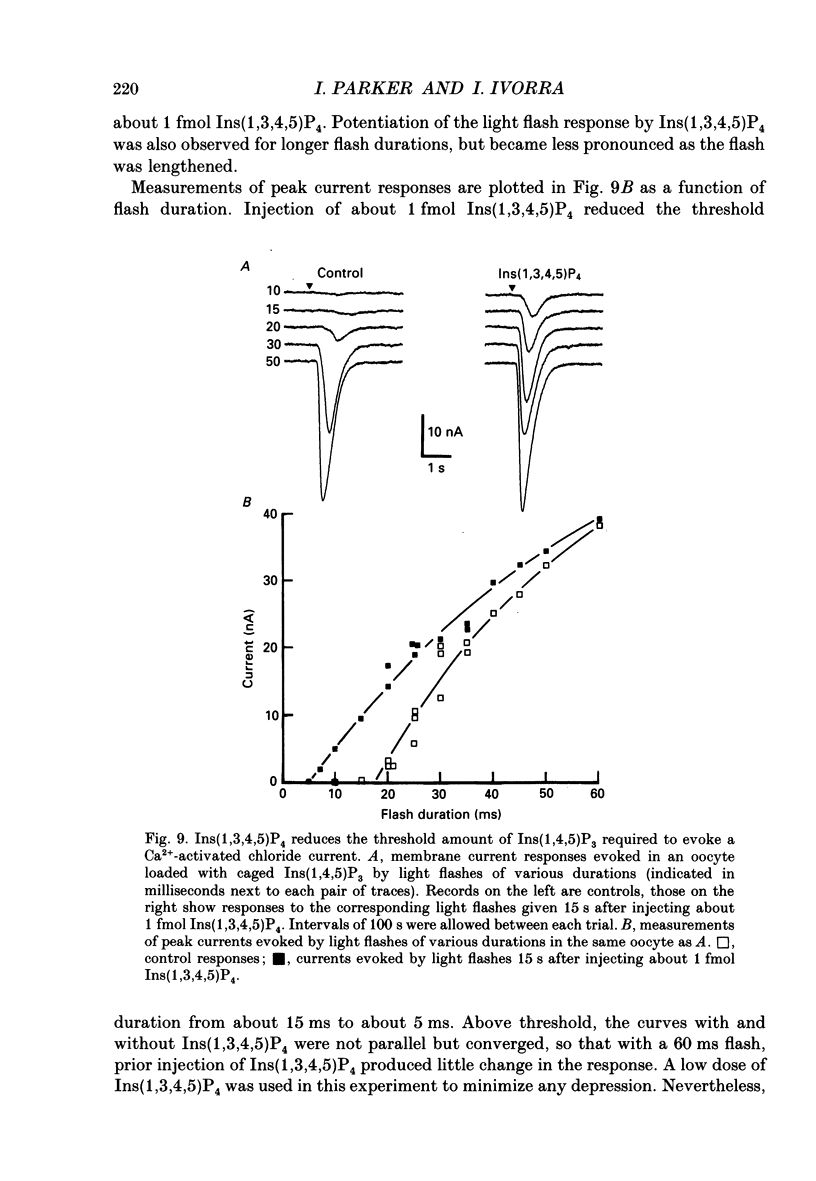
- Berridge M. J. Inositol trisphosphate-induced membrane potential oscillations in Xenopus oocytes. J Physiol. 1988 Sep;403:589–599. doi: 10.1113/jphysiol.1988.sp017266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Biden T. J., Wollheim C. B. Ca2+ regulates the inositol tris/tetrakisphosphate pathway in intact and broken preparations of insulin-secreting RINm5F cells. J Biol Chem. 1986 Sep 15;261(26):11931–11934. [PubMed] [Google Scholar]
- Bradford P. G., Irvine R. F. Specific binding sites for [3H]inositol(1,3,4,5)tetrakisphosphate on membranes of HL-60 cells. Biochem Biophys Res Commun. 1987 Dec 16;149(2):680–685. doi: 10.1016/0006-291x(87)90421-9. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., Irvine R. F., Berridge M. J., McKinney J. S., Putney J. W., Jr Actions of inositol phosphates on Ca2+ pools in guinea-pig hepatocytes. Biochem J. 1984 Dec 15;224(3):741–746. doi: 10.1042/bj2240741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changya L., Gallacher D. V., Irvine R. F., Potter B. V., Petersen O. H. Inositol 1,3,4,5-tetrakisphosphate is essential for sustained activation of the Ca2+-dependent K+ current in single internally perfused mouse lacrimal acinar cells. J Membr Biol. 1989 Jul;109(1):85–93. doi: 10.1007/BF01870793. [DOI] [PubMed] [Google Scholar]
- Connolly T. M., Bansal V. S., Bross T. E., Irvine R. F., Majerus P. W. The metabolism of tris- and tetraphosphates of inositol by 5-phosphomonoesterase and 3-kinase enzymes. J Biol Chem. 1987 Feb 15;262(5):2146–2149. [PubMed] [Google Scholar]
- Crossley I., Swann K., Chambers E., Whitaker M. Activation of sea urchin eggs by inositol phosphates is independent of external calcium. Biochem J. 1988 May 15;252(1):257–262. doi: 10.1042/bj2520257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen P. J., Irvine R. F., Drøbak B. K., Dawson A. P. Inositol 1,3,4,5-tetrakisphosphate causes release of Ca2+ from permeabilized mouse lymphoma L1210 cells by its conversion into inositol 1,4,5-trisphosphate. Biochem J. 1989 May 1;259(3):931–933. doi: 10.1042/bj2590931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Enyedi P., Williams G. H. Heterogenous inositol tetrakisphosphate binding sites in the adrenal cortex. J Biol Chem. 1988 Jun 15;263(17):7940–7942. [PubMed] [Google Scholar]
- Gillo B., Lass Y., Nadler E., Oron Y. The involvement of inositol 1,4,5-trisphosphate and calcium in the two-component response to acetylcholine in Xenopus oocytes. J Physiol. 1987 Nov;392:349–361. doi: 10.1113/jphysiol.1987.sp016784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins P. T., Stephens L., Downes C. P. Rapid formation of inositol 1,3,4,5-tetrakisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands may both result indirectly from receptor-stimulated release of inositol 1,4,5-trisphosphate from phosphatidylinositol 4,5-bisphosphate. Biochem J. 1986 Sep 1;238(2):507–516. doi: 10.1042/bj2380507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F. 'Quantal' Ca2+ release and the control of Ca2+ entry by inositol phosphates--a possible mechanism. FEBS Lett. 1990 Apr 9;263(1):5–9. doi: 10.1016/0014-5793(90)80692-c. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Heslop J. P., Berridge M. J. The inositol tris/tetrakisphosphate pathway--demonstration of Ins(1,4,5)P3 3-kinase activity in animal tissues. Nature. 1986 Apr 17;320(6063):631–634. doi: 10.1038/320631a0. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Lander D. J., Berridge M. J. Specificity of inositol phosphate-stimulated Ca2+ mobilization from Swiss-mouse 3T3 cells. Biochem J. 1986 Nov 15;240(1):301–304. doi: 10.1042/bj2400301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M. Inositol(1,3,4,5)tetrakisphosphate-induced activation of sea urchin eggs requires the presence of inositol trisphosphate. Biochem Biophys Res Commun. 1987 Jul 15;146(1):284–290. doi: 10.1016/0006-291x(87)90723-6. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M. Micro-injection of inositol 1,3,4,5-tetrakisphosphate activates sea urchin eggs by a mechanism dependent on external Ca2+. Biochem J. 1986 Dec 15;240(3):917–920. doi: 10.1042/bj2400917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M., Pollock W. K., Smith P. M., Wreggett K. A. Inositol phosphates: proliferation, metabolism and function. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):281–298. doi: 10.1098/rstb.1988.0077. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Hansen C. A., Williamson J. R. Inositol tetrakisphosphate mobilizes calcium from cerebellum microsomes. Mol Pharmacol. 1989 Sep;36(3):391–397. [PubMed] [Google Scholar]
- Joseph S. K., Williamson J. R. Inositol polyphosphates and intracellular calcium release. Arch Biochem Biophys. 1989 Aug 15;273(1):1–15. doi: 10.1016/0003-9861(89)90156-2. [DOI] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J Physiol. 1982 Jul;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I. Chloride current induced by injection of calcium into Xenopus oocytes. J Physiol. 1984 Dec;357:173–183. doi: 10.1113/jphysiol.1984.sp015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I. Latencies of membrane currents evoked in Xenopus oocytes by receptor activation, inositol trisphosphate and calcium. J Physiol. 1989 Aug;415:189–210. doi: 10.1113/jphysiol.1989.sp017718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minta A., Kao J. P., Tsien R. Y. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J Biol Chem. 1989 May 15;264(14):8171–8178. [PubMed] [Google Scholar]
- Morris A. P., Gallacher D. V., Irvine R. F., Petersen O. H. Synergism of inositol trisphosphate and tetrakisphosphate in activating Ca2+-dependent K+ channels. Nature. 1987 Dec 17;330(6149):653–655. doi: 10.1038/330653a0. [DOI] [PubMed] [Google Scholar]
- Oron Y., Dascal N., Nadler E., Lupu M. Inositol 1,4,5-trisphosphate mimics muscarinic response in Xenopus oocytes. Nature. 1985 Jan 10;313(5998):141–143. doi: 10.1038/313141a0. [DOI] [PubMed] [Google Scholar]
- Parker I., Ivorra I. Inhibition by Ca2+ of inositol trisphosphate-mediated Ca2+ liberation: a possible mechanism for oscillatory release of Ca2+. Proc Natl Acad Sci U S A. 1990 Jan;87(1):260–264. doi: 10.1073/pnas.87.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Miledi R. Changes in intracellular calcium and in membrane currents evoked by injection of inositol trisphosphate into Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1986 Aug 22;228(1252):307–315. doi: 10.1098/rspb.1986.0057. [DOI] [PubMed] [Google Scholar]
- Parker I., Miledi R. Injection of inositol 1,3,4,5-tetrakisphosphate into Xenopus oocytes generates a chloride current dependent upon intracellular calcium. Proc R Soc Lond B Biol Sci. 1987 Oct 22;232(1266):59–70. doi: 10.1098/rspb.1987.0061. [DOI] [PubMed] [Google Scholar]
- Parker I., Miledi R. Nonlinearity and facilitation in phosphoinositide signaling studied by the use of caged inositol trisphosphate in Xenopus oocytes. J Neurosci. 1989 Nov;9(11):4068–4077. doi: 10.1523/JNEUROSCI.09-11-04068.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne R., Walz B., Levy S., Fein A. The localization of calcium release by inositol trisphosphate in Limulus photoreceptors and its control by negative feedback. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):359–379. doi: 10.1098/rstb.1988.0082. [DOI] [PubMed] [Google Scholar]
- Pittet D., Schlegel W., Lew D. P., Monod A., Mayr G. W. Mass changes in inositol tetrakis- and pentakisphosphate isomers induced by chemotactic peptide stimulation in HL-60 cells. J Biol Chem. 1989 Nov 5;264(31):18489–18493. [PubMed] [Google Scholar]
- Rana R. S., Sekar M. C., Hokin L. E., MacDonald M. J. A possible role for glucose metabolites in the regulation of inositol-1,4,5-trisphosphate 5-phosphomonoesterase activity in pancreatic islets. J Biol Chem. 1986 Apr 25;261(12):5237–5240. [PubMed] [Google Scholar]
- Snyder P. M., Krause K. H., Welsh M. J. Inositol trisphosphate isomers, but not inositol 1,3,4,5-tetrakisphosphate, induce calcium influx in Xenopus laevis oocytes. J Biol Chem. 1988 Aug 15;263(23):11048–11051. [PubMed] [Google Scholar]
- Stith B. J., Proctor W. R. Microinjection of inositol 1,2-(cyclic)-4,5-trisphosphate, inositol 1,3,4,5-tetrakisphosphate, and inositol 1,4,5-trisphosphate into intact Xenopus oocytes can induce membrane currents independent of extracellular calcium. J Cell Biochem. 1989 Jul;40(3):321–330. doi: 10.1002/jcb.240400308. [DOI] [PubMed] [Google Scholar]
- Theibert A. B., Supattapone S., Worley P. F., Baraban J. M., Meek J. L., Snyder S. H. Demonstration of inositol 1,3,4,5-tetrakisphosphate receptor binding. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1283–1289. doi: 10.1016/s0006-291x(87)80272-3. [DOI] [PubMed] [Google Scholar]


