Abstract
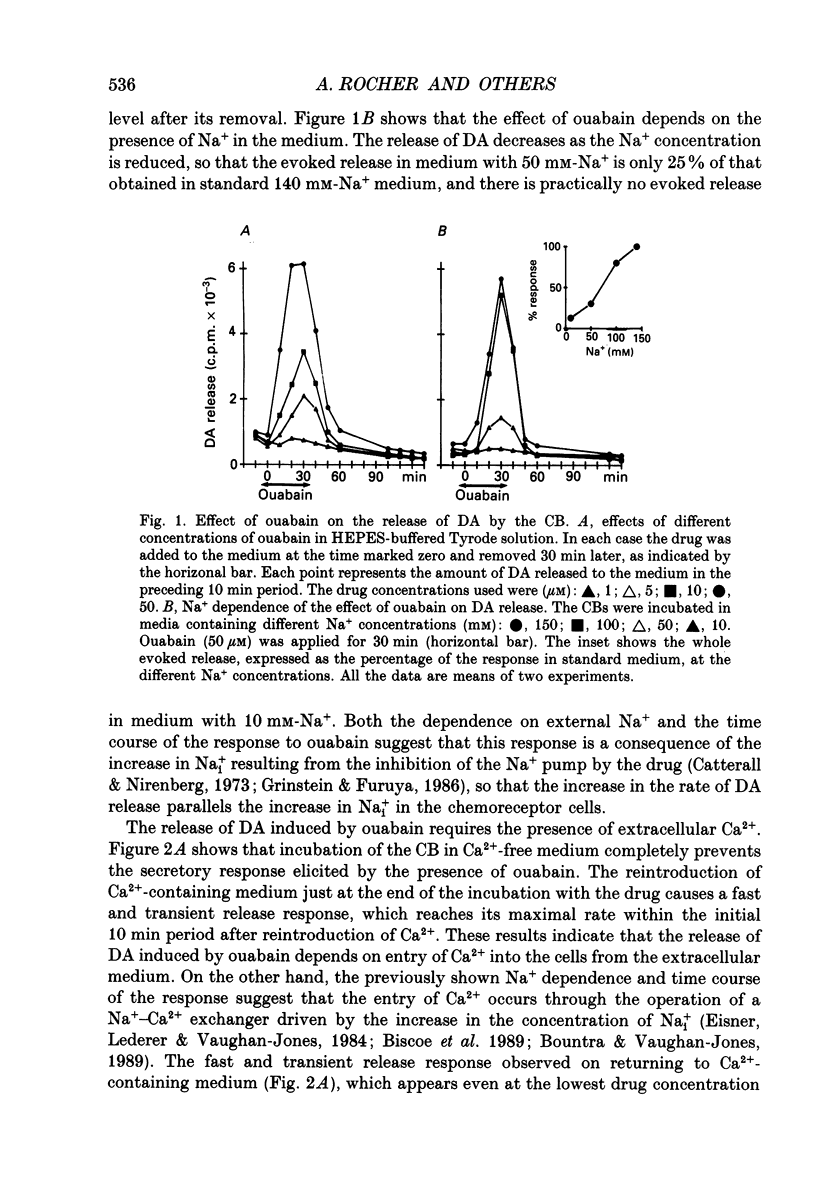
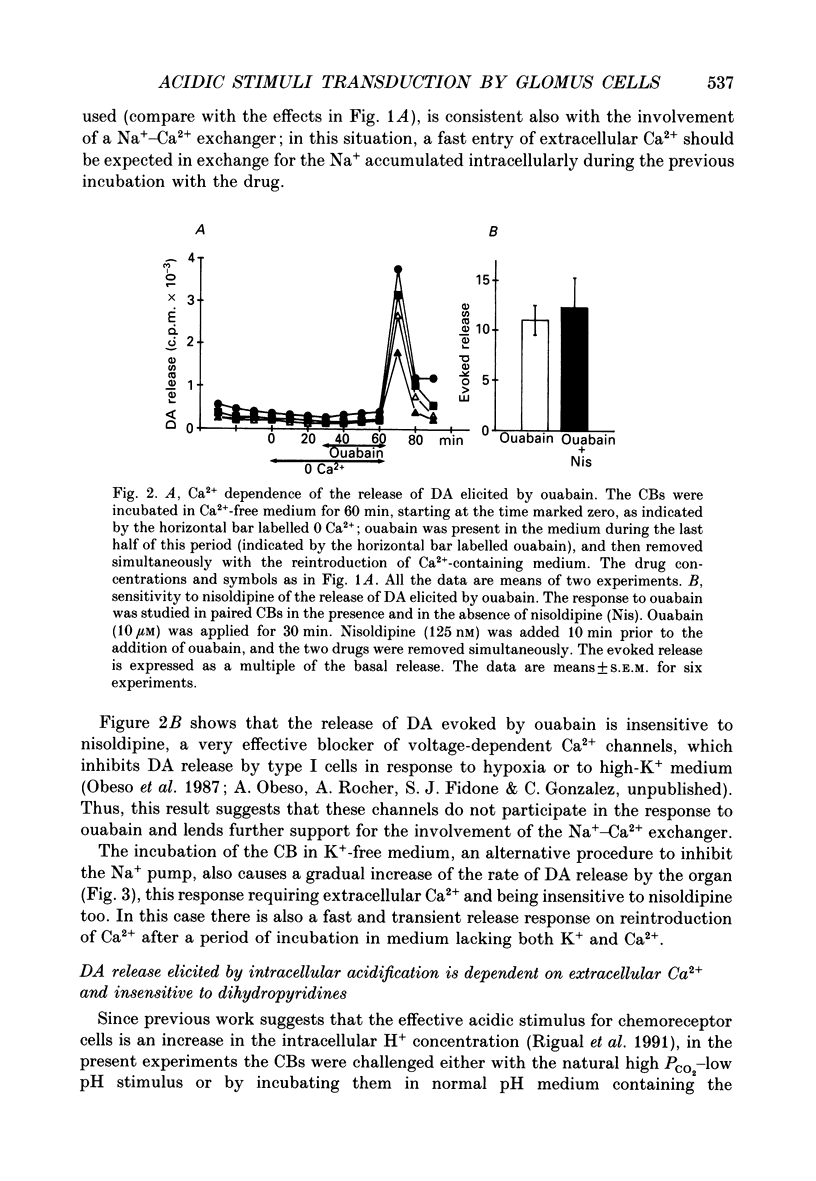
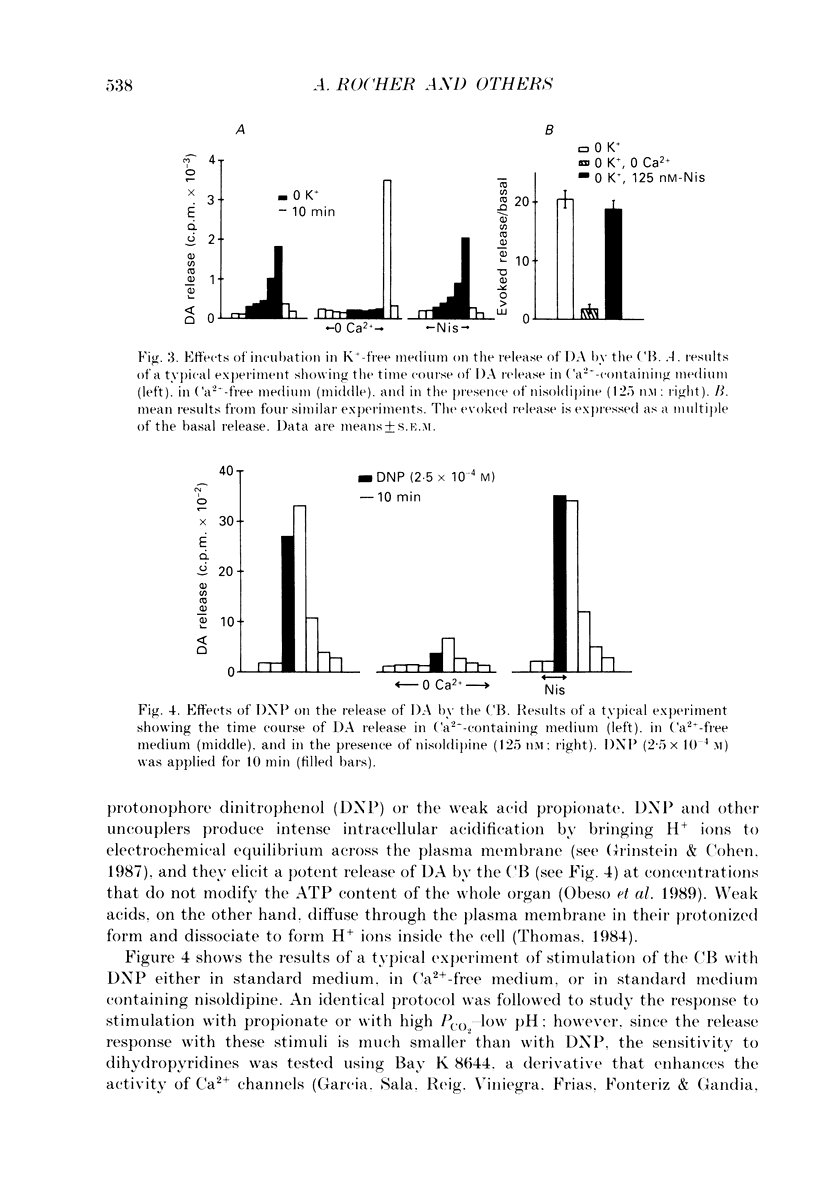
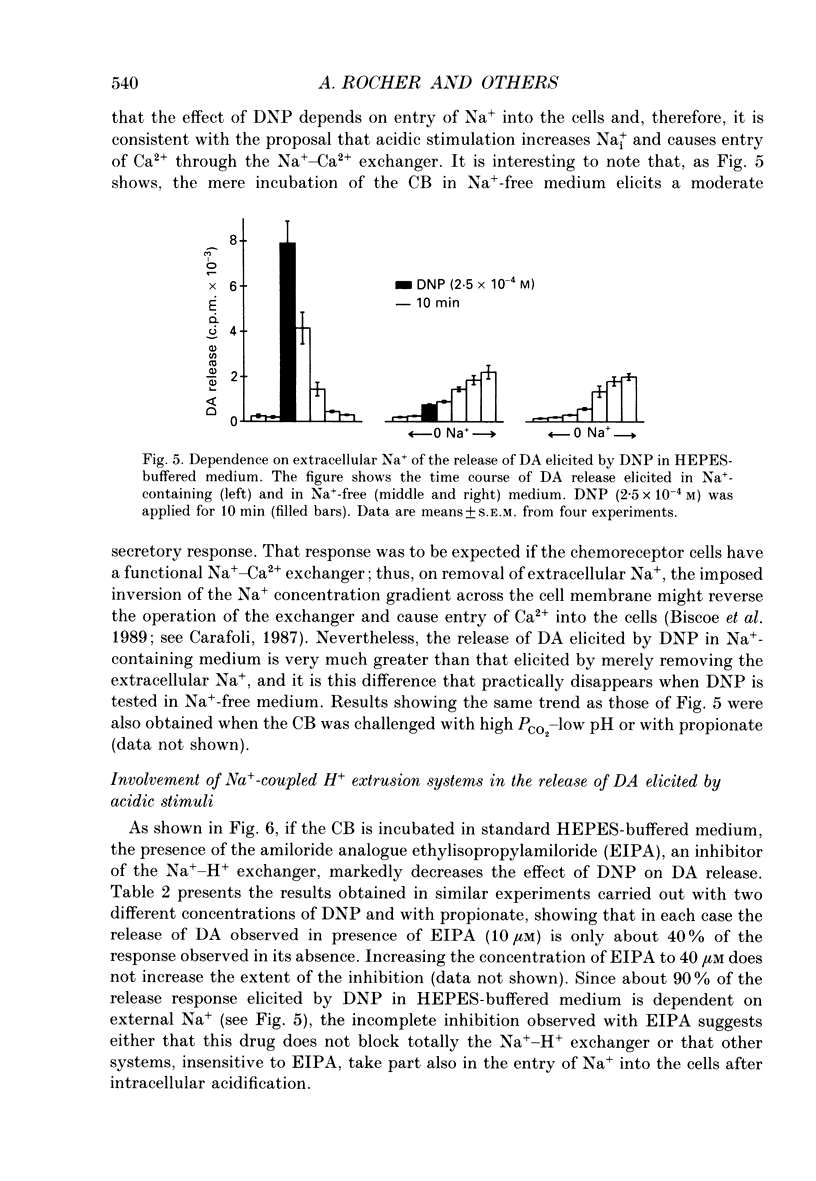
1. The release of [3H]dopamine (DA) in response to inhibition of the Na+ pump or to intracellular acid load was studied in rabbit carotid bodies (CB) previously incubated with the precursor [3H]tyrosine. The ionic requirements of the release response and the involvement of specific ion transport systems were investigated. 2. Inhibition of the Na+ pump, by incubating the CB with ouabain or in K(+)-free medium, evokes a DA release response which requires the presence of Na+ and Ca2+ in the medium and is insensitive to nisoldipine. This suggests that the response is triggered by entry of external Ca2+ through Na(+)-Ca2+ exchange, a consequence of the increase in intracellular Na+ resulting from inhibition of the pump. 3. Incubation of the CB in medium equilibrated with 20% CO2 at pH 6.6, or in medium containing the protonophore dinitrophenol (DNP) or the weak acid propionate, elicits a DA release response which requires also the presence of Na+ and Ca2+ in the medium and is insensitive to dihydropyridines. 4. Ethylisopropylamiloride (EIPA), an inhibitor of the Na(+)-H+ exchanger, markedly decreases the release response elicited by DNP or propionate in bicarbonate-free medium, but has not any effect in bicarbonate-buffered medium. In the latter condition, the EIPA-insensitive release of DA is inhibited by reducing the HCO3- concentration in the medium to 2 mM or by removal of Cl-, suggesting that in bicarbonate-buffered medium a Na(+)-dependent HCO3(-)-Cl- exchanger is involved in the release response. 5. It is concluded that the release of DA by the chemoreceptor cells in response to acidic stimulation is triggered by entry of external Ca2+ through Na(+)-Ca2+ exchange. This exchange is promoted by the increase of intracellular Na+ that results from the operation of Na(+)-coupled H(+)-extruding mechanisms activated by the acid load.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almaraz L., Gonzalez C., Obeso A. Effects of high potassium on the release of [3H]dopamine from the cat carotid body in vitro. J Physiol. 1986 Oct;379:293–307. doi: 10.1113/jphysiol.1986.sp016254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierman A. J., Cragoe E. J., Jr, de Laat S. W., Moolenaar W. H. Bicarbonate determines cytoplasmic pH and suppresses mitogen-induced alkalinization in fibroblastic cells. J Biol Chem. 1988 Oct 25;263(30):15253–15256. [PubMed] [Google Scholar]
- Biscoe T. J., Duchen M. R., Eisner D. A., O'Neill S. C., Valdeolmillos M. Measurements of intracellular Ca2+ in dissociated type I cells of the rabbit carotid body. J Physiol. 1989 Sep;416:421–434. doi: 10.1113/jphysiol.1989.sp017769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Duchen M. R. Electrophysiological responses of dissociated type I cells of the rabbit carotid body to cyanide. J Physiol. 1989 Jun;413:447–468. doi: 10.1113/jphysiol.1989.sp017663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P. Calcium transport and buffering in neurons. Trends Neurosci. 1988 Oct;11(10):438–443. doi: 10.1016/0166-2236(88)90195-6. [DOI] [PubMed] [Google Scholar]
- Bountra C., Vaughan-Jones R. D. Effect of intracellular and extracellular pH on contraction in isolated, mammalian cardiac muscle. J Physiol. 1989 Nov;418:163–187. doi: 10.1113/jphysiol.1989.sp017833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Catterall W. A., Nirenberg M. Sodium uptake associated with activation of action potential ionophores of cultured neuroblastoma and muscle cells. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3759–3763. doi: 10.1073/pnas.70.12.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpiano M. A., Hescheler J. Evidence for a PO2-sensitive K+ channel in the type-I cell of the rabbit carotid body. FEBS Lett. 1989 Jun 5;249(2):195–198. doi: 10.1016/0014-5793(89)80623-4. [DOI] [PubMed] [Google Scholar]
- Duchen M. R., Caddy K. W., Kirby G. C., Patterson D. L., Ponte J., Biscoe T. J. Biophysical studies of the cellular elements of the rabbit carotid body. Neuroscience. 1988 Jul;26(1):291–311. doi: 10.1016/0306-4522(88)90146-7. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J., Vaughan-Jones R. D. The quantitative relationship between twitch tension and intracellular sodium activity in sheep cardiac Purkinje fibres. J Physiol. 1984 Oct;355:251–266. doi: 10.1113/jphysiol.1984.sp015417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidone S., Gonzalez C., Yoshizaki K. Effects of low oxygen on the release of dopamine from the rabbit carotid body in vitro. J Physiol. 1982 Dec;333:93–110. doi: 10.1113/jphysiol.1982.sp014441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz M. B., Boyarsky G., Sterzel R. B., Boron W. F. Arginine vasopressin enhances pHi regulation in the presence of HCO3- by stimulating three acid-base transport systems. Nature. 1989 Feb 16;337(6208):648–651. doi: 10.1038/337648a0. [DOI] [PubMed] [Google Scholar]
- García A. G., Sala F., Reig J. A., Viniegra S., Frías J., Fontériz R., Gandía L. Dihydropyridine BAY-K-8644 activates chromaffin cell calcium channels. Nature. 1984 May 3;309(5963):69–71. doi: 10.1038/309069a0. [DOI] [PubMed] [Google Scholar]
- Gonzalez E., Rigual R., Fidone S. J., Gonzalez C. Mechanisms for termination of the action of dopamine in carotid body chemoreceptors. J Auton Nerv Syst. 1987 Mar;18(3):249–259. doi: 10.1016/0165-1838(87)90123-8. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Cohen S. Cytoplasmic [Ca2+] and intracellular pH in lymphocytes. Role of membrane potential and volume-activated Na+/H+ exchange. J Gen Physiol. 1987 Feb;89(2):185–213. doi: 10.1085/jgp.89.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Furuya W. Characterization of the amiloride-sensitive Na+-H+ antiport of human neutrophils. Am J Physiol. 1986 Feb;250(2 Pt 1):C283–C291. doi: 10.1152/ajpcell.1986.250.2.C283. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Rothstein A. Mechanisms of regulation of the Na+/H+ exchanger. J Membr Biol. 1986;90(1):1–12. doi: 10.1007/BF01869680. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Rotin D., Mason M. J. Na+/H+ exchange and growth factor-induced cytosolic pH changes. Role in cellular proliferation. Biochim Biophys Acta. 1989 Jan 18;988(1):73–97. doi: 10.1016/0304-4157(89)90004-x. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Delpiano M. A., Acker H., Pietruschka F. Ionic currents on type-I cells of the rabbit carotid body measured by voltage-clamp experiments and the effect of hypoxia. Brain Res. 1989 May 1;486(1):79–88. doi: 10.1016/0006-8993(89)91280-8. [DOI] [PubMed] [Google Scholar]
- L'Allemain G., Paris S., Pouysségur J. Role of a Na+-dependent Cl-/HCO3- exchange in regulation of intracellular pH in fibroblasts. J Biol Chem. 1985 Apr 25;260(8):4877–4883. [PubMed] [Google Scholar]
- López-Barneo J., López-López J. R., Ureña J., González C. Chemotransduction in the carotid body: K+ current modulated by PO2 in type I chemoreceptor cells. Science. 1988 Jul 29;241(4865):580–582. doi: 10.1126/science.2456613. [DOI] [PubMed] [Google Scholar]
- López-López J., González C., Ureña J., López-Barneo J. Low pO2 selectively inhibits K channel activity in chemoreceptor cells of the mammalian carotid body. J Gen Physiol. 1989 May;93(5):1001–1015. doi: 10.1085/jgp.93.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso A., Almaraz L., Gonzalez C. Effects of 2-deoxy-D-glucose on in vitro cat carotid body. Brain Res. 1986 Apr 16;371(1):25–36. doi: 10.1016/0006-8993(86)90806-1. [DOI] [PubMed] [Google Scholar]
- Obeso A., Almaraz L., Gonzalez C. Effects of cyanide and uncouplers on chemoreceptor activity and ATP content of the cat carotid body. Brain Res. 1989 Mar 6;481(2):250–257. doi: 10.1016/0006-8993(89)90801-9. [DOI] [PubMed] [Google Scholar]
- Renner E. L., Lake J. R., Cragoe E. J., Jr, Scharschmidt B. F. Amiloride and amiloride analogs inhibit Na+/K+-transporting ATPase and Na+-coupled alanine transport in rat hepatocytes. Biochim Biophys Acta. 1988 Mar 3;938(3):386–394. doi: 10.1016/0005-2736(88)90136-8. [DOI] [PubMed] [Google Scholar]
- Rigual R., Iñiguez C., Carreres J., Gonzalez C. Carbonic anhydrase in the carotid body and the carotid sinus nerve. Histochemistry. 1985;82(6):577–580. doi: 10.1007/BF00489979. [DOI] [PubMed] [Google Scholar]
- Rigual R., López-López J. R., Gonzalez C. Release of dopamine and chemoreceptor discharge induced by low pH and high PCO2 stimulation of the cat carotid body. J Physiol. 1991 Feb;433:519–531. doi: 10.1113/jphysiol.1991.sp018441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Experimental displacement of intracellular pH and the mechanism of its subsequent recovery. J Physiol. 1984 Sep;354:3P–22P. doi: 10.1113/jphysiol.1984.sp015397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. The role of bicarbonate, chloride and sodium ions in the regulation of intracellular pH in snail neurones. J Physiol. 1977 Dec;273(1):317–338. doi: 10.1113/jphysiol.1977.sp012096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ureña J., López-López J., González C., López-Barneo J. Ionic currents in dispersed chemoreceptor cells of the mammalian carotid body. J Gen Physiol. 1989 May;93(5):979–999. doi: 10.1085/jgp.93.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]


