Abstract
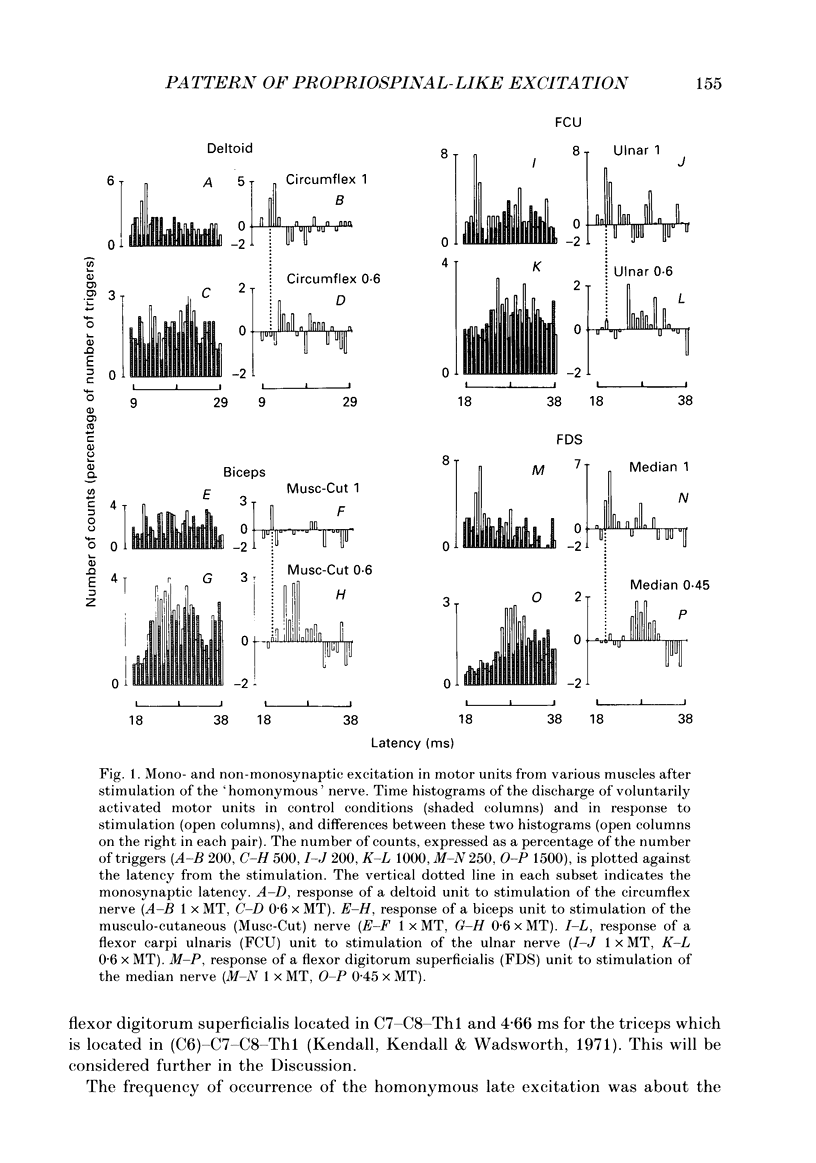
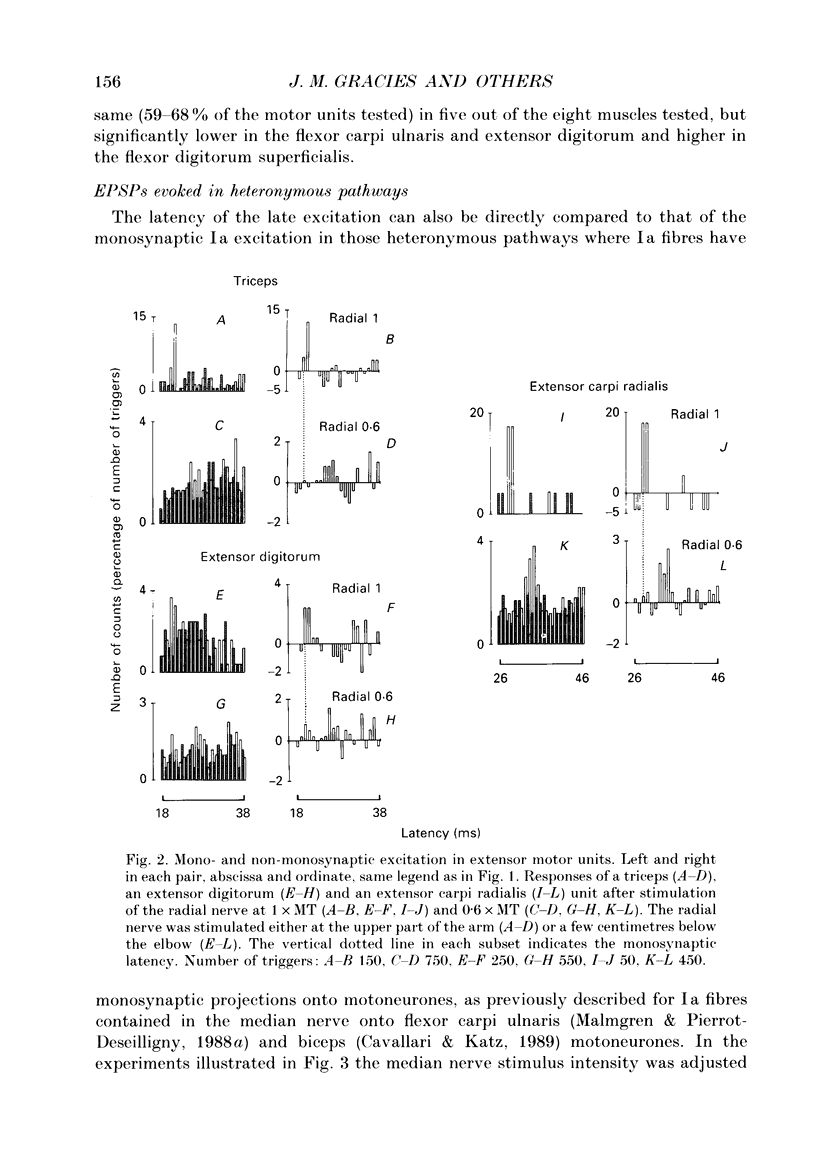
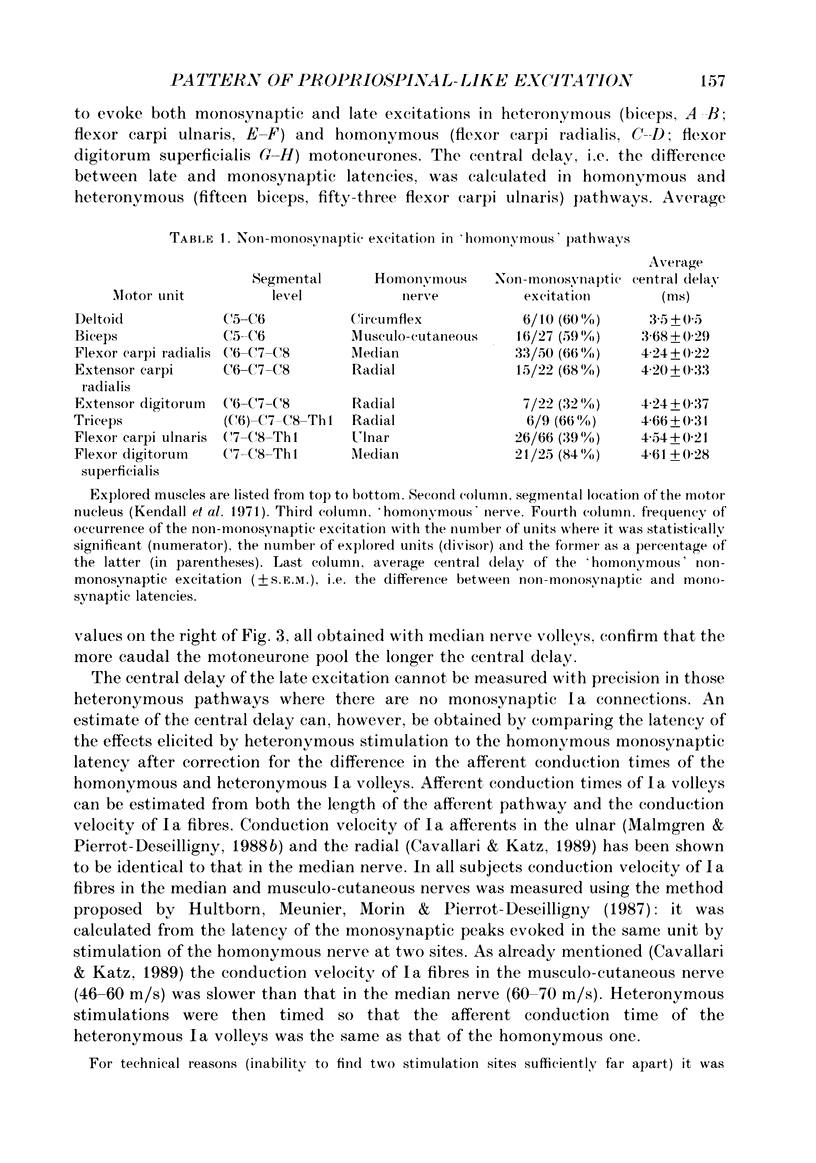
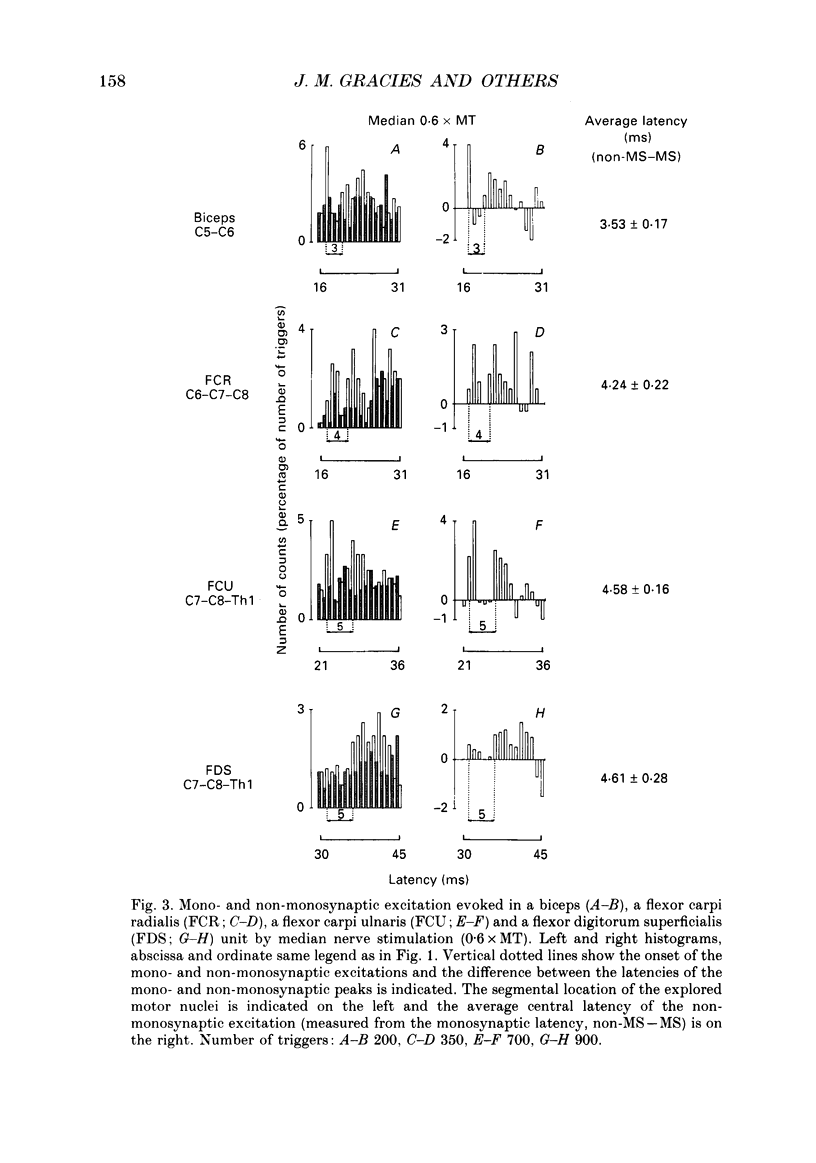
1. The pattern of distribution of non-monosynaptic (propriospinal-like) excitation to various motor nuclei (deltoid, extensors and flexors of the elbow, the wrist and the fingers) was investigated. 2. Changes in the firing probability of individual voluntarily activated motor units were studied following conditioning stimuli. Conditioning volleys were evoked by weak electrical stimuli applied to various mixed nerves (circumflex, musculocutaneous, median, radial, ulnar) and to the skin. 3. In all investigated nuclei stimulation of the 'homonymous' nerve evoked a peak of increased firing probability with a latency which was 2-7 ms longer than the monosynaptic Ia latency. The average central delay of the late excitation, measured from monosynaptic latency, seems to depend only on the segmental level of the motor nucleus: the more caudal the nucleus the longer the latency. This strongly suggest a transmission through neurones located above the cervical enlargement, as are C3--C4 propriospinal neurones in the cat. 4. Both group I muscle and cutaneous afferents were shown to contribute to propriospinal-like excitation. It is argued that a spatial facilitation of the effects evoked by these two inputs might explain why the threshold of late excitation is always below that of the monosynaptic Ia excitation in motoneurones. 5. The pattern of distribution of propriospinal-like excitation was diffuse: stimulation of each mixed nerve was able to evoke excitation in all investigated motor nuclei. Similarly, stimulation of a given skin field could produce excitation of biceps and wrist flexor and extensor units. 6. Each motor nucleus therefore receives excitation from a multimodal and wide range peripheral input. However, it is argued that what appears as a diffuse pattern might simply reflect connections which are not used in each movement but appropriately selected by higher centres.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alstermark B., Lundberg A., Norrsell U., Sybirska E. Integration in descending motor pathways controlling the forelimb in the cat. 9. Differential behavioural defects after spinal cord lesions interrupting defined pathways from higher centres to motoneurones. Exp Brain Res. 1981;42(3-4):299–318. doi: 10.1007/BF00237496. [DOI] [PubMed] [Google Scholar]
- Alstermark B., Sasaki S. Integration in descending motor pathways controlling the forelimb in the cat. 15. Comparison of the projection from excitatory C3-C4 propriospinal neurones to different species of forelimb motoneurones. Exp Brain Res. 1986;63(3):543–556. doi: 10.1007/BF00237477. [DOI] [PubMed] [Google Scholar]
- Baldissera F., Pierrot-Deseilligny E. Facilitation of transmission in the pathway of non-monosynaptic Ia excitation to wrist flexor motoneurones at the onset of voluntary movement in man. Exp Brain Res. 1989;74(2):437–439. doi: 10.1007/BF00248880. [DOI] [PubMed] [Google Scholar]
- Burke D., Mackenzie R. A., Skuse N. F., Lethlean A. K. Cutaneous afferent activity in median and radial nerve fascicles: a microelectrode study. J Neurol Neurosurg Psychiatry. 1975 Sep;38(9):855–864. doi: 10.1136/jnnp.38.9.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallari P., Katz R. Pattern of projections of group I afferents from forearm muscles to motoneurones supplying biceps and triceps muscles in man. Exp Brain Res. 1989;78(3):465–478. doi: 10.1007/BF00230235. [DOI] [PubMed] [Google Scholar]
- Fetz E. E., Gustafsson B. Relation between shapes of post-synaptic potentials and changes in firing probability of cat motoneurones. J Physiol. 1983 Aug;341:387–410. doi: 10.1113/jphysiol.1983.sp014812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier E., Meunier S., Pierrot-Deseilligny E., Shindo M. Evidence for interneuronally mediated Ia excitatory effects to human quadriceps motoneurones. J Physiol. 1986 Aug;377:143–169. doi: 10.1113/jphysiol.1986.sp016179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Meunier S., Morin C., Pierrot-Deseilligny E. Assessing changes in presynaptic inhibition of I a fibres: a study in man and the cat. J Physiol. 1987 Aug;389:729–756. doi: 10.1113/jphysiol.1987.sp016680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illert M., Lundberg A., Padel Y., Tanaka R. Integration in descending motor pathways controlling the forelimb in the cat. 5. Properties of and monosynaptic excitatory convergence on C3--C4 propriospinal neurones. Exp Brain Res. 1978 Sep 15;33(1):101–130. doi: 10.1007/BF00238798. [DOI] [PubMed] [Google Scholar]
- Malmgren K., Pierrot-Deseilligny E. Evidence for non-monosynaptic Ia excitation of human wrist flexor motoneurones, possibly via propriospinal neurones. J Physiol. 1988 Nov;405:747–764. doi: 10.1113/jphysiol.1988.sp017359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmgren K., Pierrot-Deseilligny E. Inhibition of neurones transmitting non-monosynaptic Ia excitation to human wrist flexor motoneurones. J Physiol. 1988 Nov;405:765–783. doi: 10.1113/jphysiol.1988.sp017360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao C. C., Ashby P., Wang M., McCrea D. Synaptic connections from large muscle afferents to the motoneurons of various leg muscles in man. Exp Brain Res. 1984;56(2):341–350. doi: 10.1007/BF00236290. [DOI] [PubMed] [Google Scholar]
- Marsden C. D., Rothwell J. C., Day B. L. Long-latency automatic responses to muscle stretch in man: origin and function. Adv Neurol. 1983;39:509–539. [PubMed] [Google Scholar]
- Matthews P. B. Evidence from the use of vibration that the human long-latency stretch reflex depends upon spindle secondary afferents. J Physiol. 1984 Mar;348:383–415. doi: 10.1113/jphysiol.1984.sp015116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J., Pierrot-Deseilligny E. Pattern of cutaneous inhibition of the propriospinal-like excitation to human upper limb motoneurones. J Physiol. 1991 Mar;434:169–182. doi: 10.1113/jphysiol.1991.sp018464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosén I., Sjölund B. Organization of group I activated cells in the main and external cuneate nuclei of the cat: identification of muscle receptors. Exp Brain Res. 1973 Jan 29;16(3):221–237. doi: 10.1007/BF00233327. [DOI] [PubMed] [Google Scholar]
- Stephens J. A., Usherwood T. P., Garnett R. Technique for studying synaptic connections of single motoneurones in man. Nature. 1976 Sep 23;263(5575):343–344. doi: 10.1038/263343a0. [DOI] [PubMed] [Google Scholar]


