Abstract
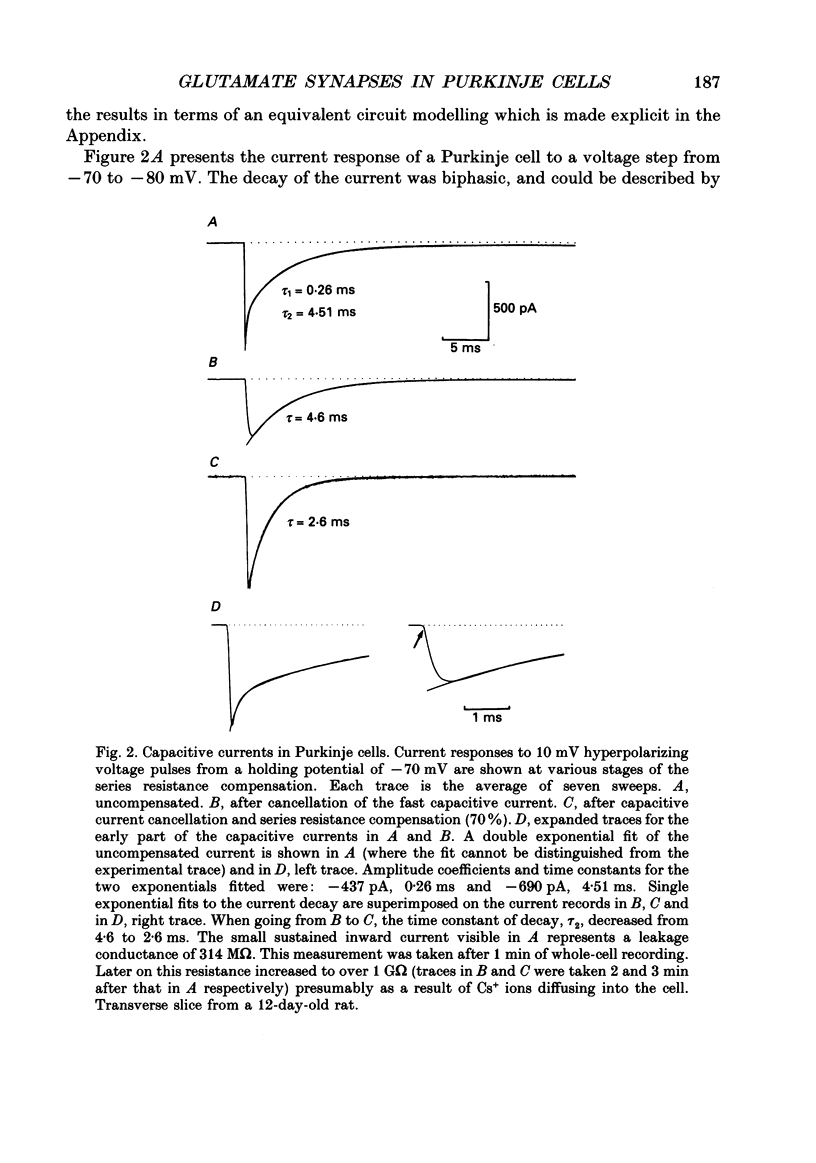
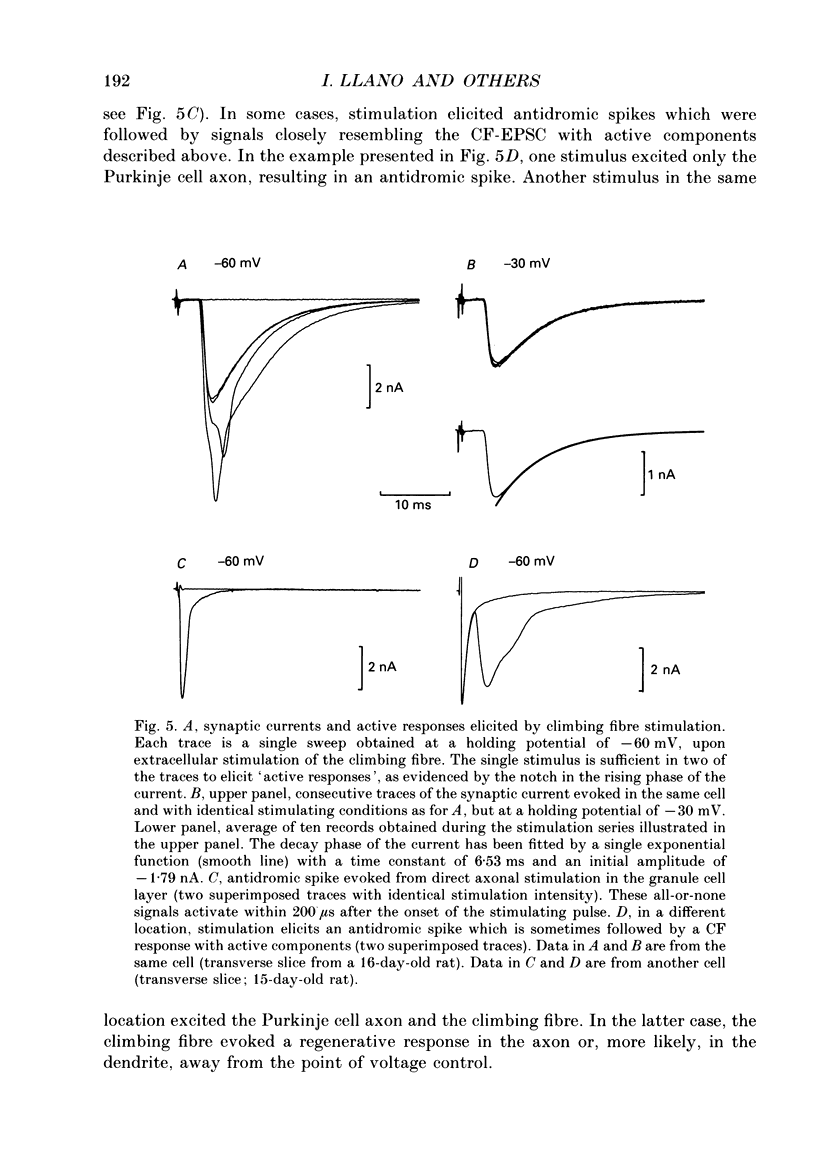
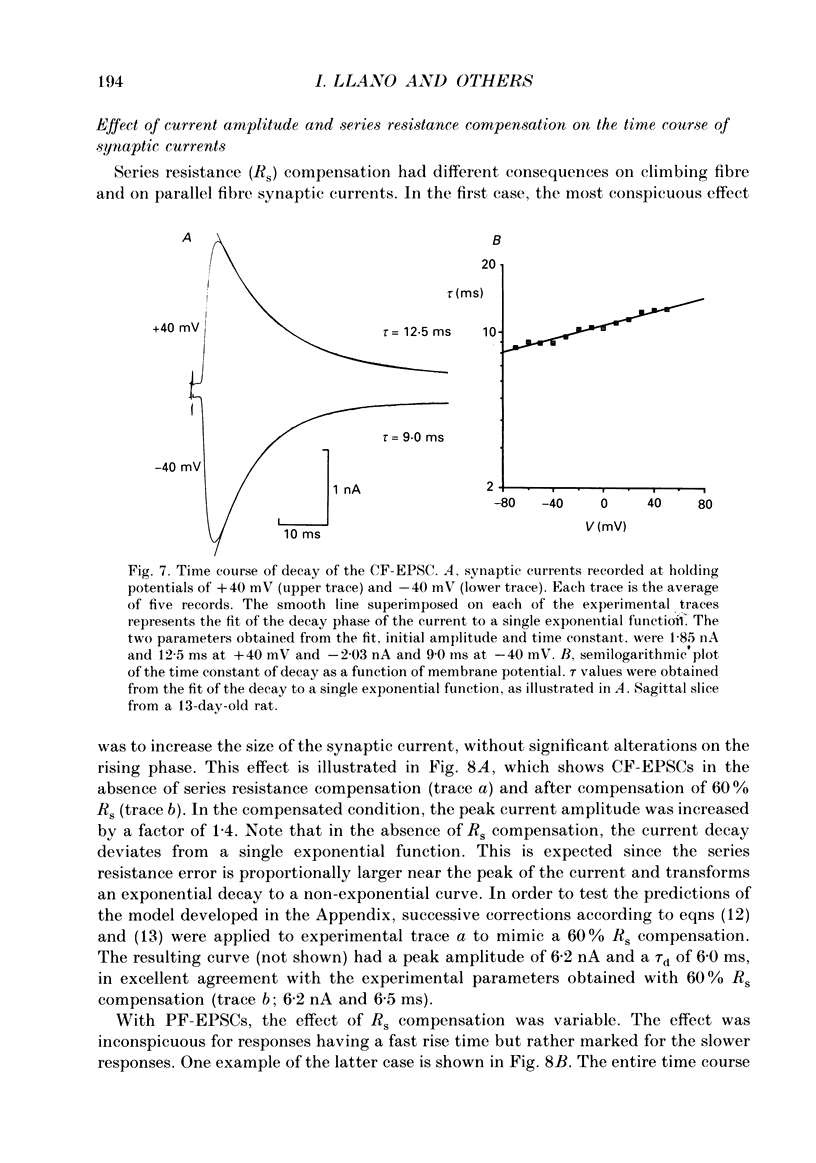
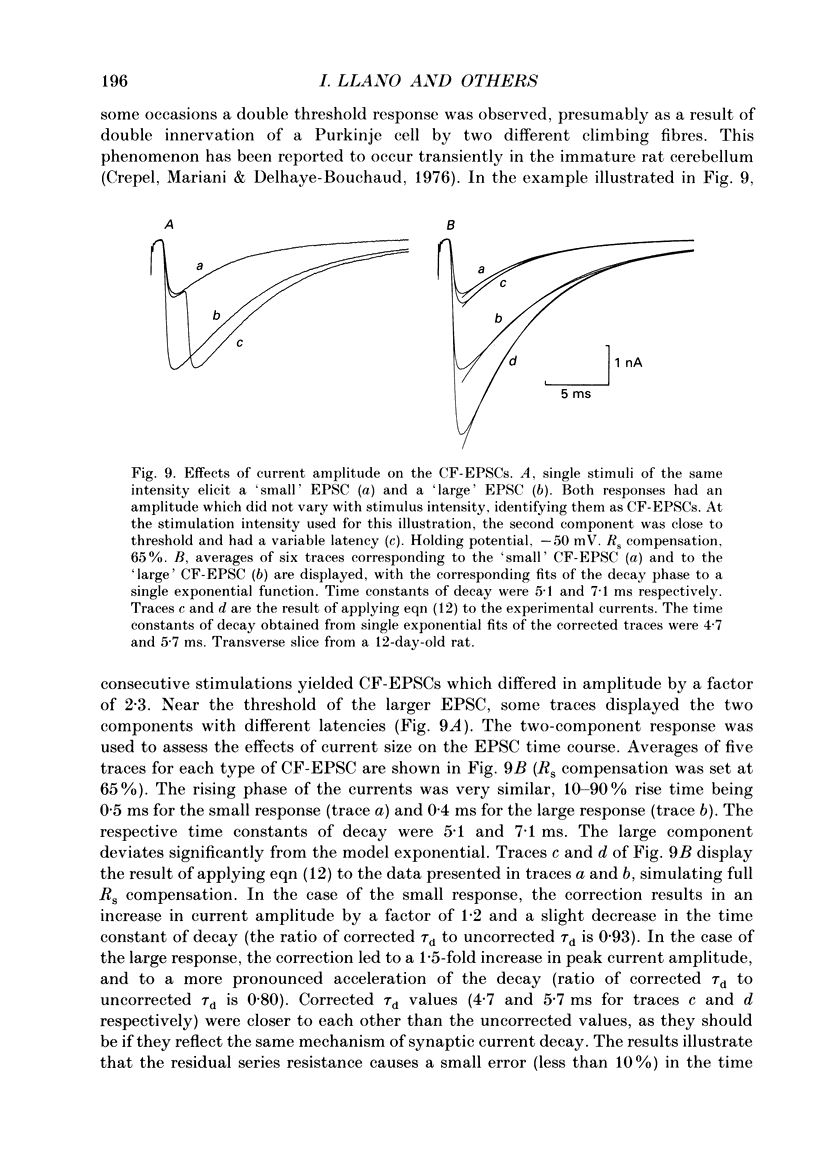
1. Postsynaptic currents originating from activation of the two major excitatory inputs to Purkinje cells were studied in thin slices of rat cerebellum, using the tight-seal whole-cell recording technique. Two types of excitatory postsynaptic currents were analysed: those evoked by stimulation of the granule cell-parallel fibre system (PF-EPSC) and those elicited by stimulation of the climbing fibres (CF-EPSC). 2. Both types of postsynaptic currents had a linear current-voltage relation, reversing at membrane potentials close to 0 mV. Their time course of activation was independent of the membrane potential. 3. For both types of postsynaptic currents, the time course of decay was well described by a single exponential function, with a time constant which increased as the membrane potential was made more positive. 4. Postsynaptic currents arising from stimulation of the climbing fibre generally had a slightly faster time course of onset and decay than those associated with stimulation of the granule cell-parallel fibre system. The average values of the 10-90% rise time were 1.8 +/- 0.4 ms (means +/- S.D., n = 7) for PF-EPSCs and 0.8 +/- 0.3 ms (n = 9) for CF-EPSCs. Time constants of decay, at a holding potential of -60 mV, had values of 8.3 +/- 1.6 ms (n = 7) and 6.4 +/- 1.1 ms (n = 9) for PF-EPSCs and CF-EPSCs respectively. 5. CF-EPSCs and PF-EPSCs had the characteristics described above in slices derived from animals aged 9-22 days old and 9-15 days old, respectively. The PF-EPSCs in animals older than 15 days had very slow time courses and positive apparent reversal potentials, suggesting that they originated from distal locations, not under accurate voltage control. 6. In order to assess the quality of the voltage clamp, responses to hyperpolarizing pulses from -70 mV were analysed. The capacitive currents could be fitted by the sum of two exponentials, and were interpreted with an equivalent electrical circuit comprising two main compartments (soma and proximal dendrites on one hand, distal dendrites on the other). Analysis of synaptic currents in terms of this model suggested that the recorded time course of decay was approximately correct. 7. CF-EPSCs as well as PF-EPSCs were insensitive to the NMDA receptor antagonist 3-3(2-carboxypiperazine-4-yl)propyl-1-phosphonate (CPP), but were blocked in a dose-dependent reversible manner by the non-NMDA antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX).(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Audinat E., Knöpfel T., Gähwiler B. H. Responses to excitatory amino acids of Purkinje cells' and neurones of the deep nuclei in cerebellar slice cultures. J Physiol. 1990 Nov;430:297–313. doi: 10.1113/jphysiol.1990.sp018292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew H., Attwell D. Electrogenic glutamate uptake is a major current carrier in the membrane of axolotl retinal glial cells. 1987 Jun 25-Jul 1Nature. 327(6124):707–709. doi: 10.1038/327707a0. [DOI] [PubMed] [Google Scholar]
- Brown T. H., Johnston D. Voltage-clamp analysis of mossy fiber synaptic input to hippocampal neurons. J Neurophysiol. 1983 Aug;50(2):487–507. doi: 10.1152/jn.1983.50.2.487. [DOI] [PubMed] [Google Scholar]
- Crepel F., Dhanjal S. S., Garthwaite J. Morphological and electrophysiological characteristics of rat cerebellar slices maintained in vitro. J Physiol. 1981 Jul;316:127–138. doi: 10.1113/jphysiol.1981.sp013777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel F., Dhanjal S. S., Sears T. A. Effect of glutamate, aspartate and related derivatives on cerebellar purkinje cell dendrites in the rat: an in vitro study. J Physiol. 1982 Aug;329:297–317. doi: 10.1113/jphysiol.1982.sp014304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel F., Dupont J. L., Gardette R. Voltage clamp analysis of the effect of excitatory amino acids and derivatives on Purkinje cell dendrites in rat cerebellar slices maintained in vitro. Brain Res. 1983 Nov 21;279(1-2):311–315. doi: 10.1016/0006-8993(83)90200-7. [DOI] [PubMed] [Google Scholar]
- Crepel F., Mariani J., Delhaye-Bouchaud N. Evidence for a multiple innervation of Purkinje cells by climbing fibers in the immature rat cerebellum. J Neurobiol. 1976 Nov;7(6):567–578. doi: 10.1002/neu.480070609. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G. Glutamate- and GABA-receptor channels at the locust nerve-muscle junction: noise analysis and single-channel recording. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):269–278. doi: 10.1101/sqb.1983.048.01.029. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Howe J. R., Ogden D. C. Noise and single channels activated by excitatory amino acids in rat cerebellar granule neurones. J Physiol. 1988 Jun;400:189–222. doi: 10.1113/jphysiol.1988.sp017117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. Multiple-conductance channels activated by excitatory amino acids in cerebellar neurons. Nature. 1987 Feb 5;325(6104):525–528. doi: 10.1038/325525a0. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. On the multiple-conductance single channels activated by excitatory amino acids in large cerebellar neurones of the rat. J Physiol. 1989 Aug;415:555–582. doi: 10.1113/jphysiol.1989.sp017736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. Whole-cell current noise produced by excitatory and inhibitory amino acids in large cerebellar neurones of the rat. J Physiol. 1989 Aug;415:533–553. doi: 10.1113/jphysiol.1989.sp017735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Evans R. H., Herrling P. L., Jones A. W., Olverman H. J., Pook P., Watkins J. C. CPP, a new potent and selective NMDA antagonist. Depression of central neuron responses, affinity for [3H]D-AP5 binding sites on brain membranes and anticonvulsant activity. Brain Res. 1986 Sep 10;382(1):169–173. doi: 10.1016/0006-8993(86)90127-7. [DOI] [PubMed] [Google Scholar]
- Dudel J. Nonlinear voltage dependence of excitatory synaptic current in crayfish muscle. Pflugers Arch. 1974;352(3):227–241. doi: 10.1007/BF00590488. [DOI] [PubMed] [Google Scholar]
- Dupont J. L., Gardette R., Crepel F. Postnatal development of the chemosensitivity of rat cerebellar Purkinje cells to excitatory amino acids. An in vitro study. Brain Res. 1987 Jul;431(1):59–68. doi: 10.1016/0165-3806(87)90195-7. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B., Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989 Sep;414(5):600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Finkel A. S., Redman S. J. The synaptic current evoked in cat spinal motoneurones by impulses in single group 1a axons. J Physiol. 1983 Sep;342:615–632. doi: 10.1113/jphysiol.1983.sp014872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe I. D., Westbrook G. L. Slow excitatory postsynaptic currents mediated by N-methyl-D-aspartate receptors on cultured mouse central neurones. J Physiol. 1988 Feb;396:515–533. doi: 10.1113/jphysiol.1988.sp016975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite G., Yamini B., Jr, Garthwaite J. Selective loss of Purkinje and granule cell responsiveness to N-methyl-D-aspartate in rat cerebellum during development. Brain Res. 1987 Dec 1;433(2):288–292. doi: 10.1016/0165-3806(87)90034-4. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Brodbelt A. R. Synaptic activation of N-methyl-D-aspartate and non-N-methyl-D-aspartate receptors in the mossy fibre pathway in adult and immature rat cerebellar slices. Neuroscience. 1989;29(2):401–412. doi: 10.1016/0306-4522(89)90067-5. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hestrin S., Nicoll R. A., Perkel D. J., Sah P. Analysis of excitatory synaptic action in pyramidal cells using whole-cell recording from rat hippocampal slices. J Physiol. 1990 Mar;422:203–225. doi: 10.1113/jphysiol.1990.sp017980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Hagiwara S. Synaptic transmission between rat cerebellar granule and Purkinje cells in dissociated cell culture: effects of excitatory-amino acid transmitter antagonists. Proc Natl Acad Sci U S A. 1988 Feb;85(3):934–938. doi: 10.1073/pnas.85.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T. Synaptic transmission between rat inferior olivary neurons and cerebellar Purkinje cells in culture. J Neurophysiol. 1990 Jan;63(1):181–189. doi: 10.1152/jn.1990.63.1.181. [DOI] [PubMed] [Google Scholar]
- Honoré T., Davies S. N., Drejer J., Fletcher E. J., Jacobsen P., Lodge D., Nielsen F. E. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988 Aug 5;241(4866):701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kano M., Kato M. Quisqualate receptors are specifically involved in cerebellar synaptic plasticity. Nature. 1987 Jan 15;325(6101):276–279. doi: 10.1038/325276a0. [DOI] [PubMed] [Google Scholar]
- Kimura H., Okamoto K., Sakai Y. Climbing and parallel fiber responses recorded intracellularly from Purkinje cell dendrites in guinea pig cerebellar slices. Brain Res. 1985 Dec 2;348(2):213–219. doi: 10.1016/0006-8993(85)90439-1. [DOI] [PubMed] [Google Scholar]
- Kimura H., Okamoto K., Sakai Y. Pharmacological evidence for L-aspartate as the neurotransmitter of cerebellar climbing fibres in the guinea-pig. J Physiol. 1985 Aug;365:103–119. doi: 10.1113/jphysiol.1985.sp015761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konnerth A., Keller B. U., Ballanyi K., Yaari Y. Voltage sensitivity of NMDA-receptor mediated postsynaptic currents. Exp Brain Res. 1990;81(1):209–212. doi: 10.1007/BF00230117. [DOI] [PubMed] [Google Scholar]
- Konnerth A., Llano I., Armstrong C. M. Synaptic currents in cerebellar Purkinje cells. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2662–2665. doi: 10.1073/pnas.87.7.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupa M., Crepel F. Transient Sensitivity of Rat Cerebellar Purkinje Cells to N-methyl-D-aspartate during Development. A Voltage Clamp Study in in vitro Slices. Eur J Neurosci. 1990;2(4):312–316. doi: 10.1111/j.1460-9568.1990.tb00423.x. [DOI] [PubMed] [Google Scholar]
- Llano I., Marty A., Johnson J. W., Ascher P., Gähwiler B. H. Patch-clamp recording of amino acid-activated responses in "organotypic" slice cultures. Proc Natl Acad Sci U S A. 1988 May;85(9):3221–3225. doi: 10.1073/pnas.85.9.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Nicholson C. Reversal properties of climbing fiber potential in cat Purkinje cells: an example of a distributed synapse. J Neurophysiol. 1976 Mar;39(2):311–323. doi: 10.1152/jn.1976.39.2.311. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980 Aug;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Nelson P. G., Pun R. Y., Westbrook G. L. Synaptic excitation in cultures of mouse spinal cord neurones: receptor pharmacology and behaviour of synaptic currents. J Physiol. 1986 Mar;372:169–190. doi: 10.1113/jphysiol.1986.sp016003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Shelton D. P. Membrane resistivity estimated for the Purkinje neuron by means of a passive computer model. Neuroscience. 1985 Jan;14(1):111–131. doi: 10.1016/0306-4522(85)90168-x. [DOI] [PubMed] [Google Scholar]
- Sotelo C. Purkinje cell ontogeny: formation and maintenance of spines. Prog Brain Res. 1978;48:149–170. doi: 10.1016/S0079-6123(08)61021-3. [DOI] [PubMed] [Google Scholar]
- Tang C. M., Dichter M., Morad M. Quisqualate activates a rapidly inactivating high conductance ionic channel in hippocampal neurons. Science. 1989 Mar 17;243(4897):1474–1477. doi: 10.1126/science.2467378. [DOI] [PubMed] [Google Scholar]
- Trussell L. O., Fischbach G. D. Glutamate receptor desensitization and its role in synaptic transmission. Neuron. 1989 Aug;3(2):209–218. doi: 10.1016/0896-6273(89)90034-2. [DOI] [PubMed] [Google Scholar]
- Trussell L. O., Thio L. L., Zorumski C. F., Fischbach G. D. Rapid desensitization of glutamate receptors in vertebrate central neurons. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4562–4566. doi: 10.1073/pnas.85.12.4562-a. [DOI] [PMC free article] [PubMed] [Google Scholar]




