Abstract
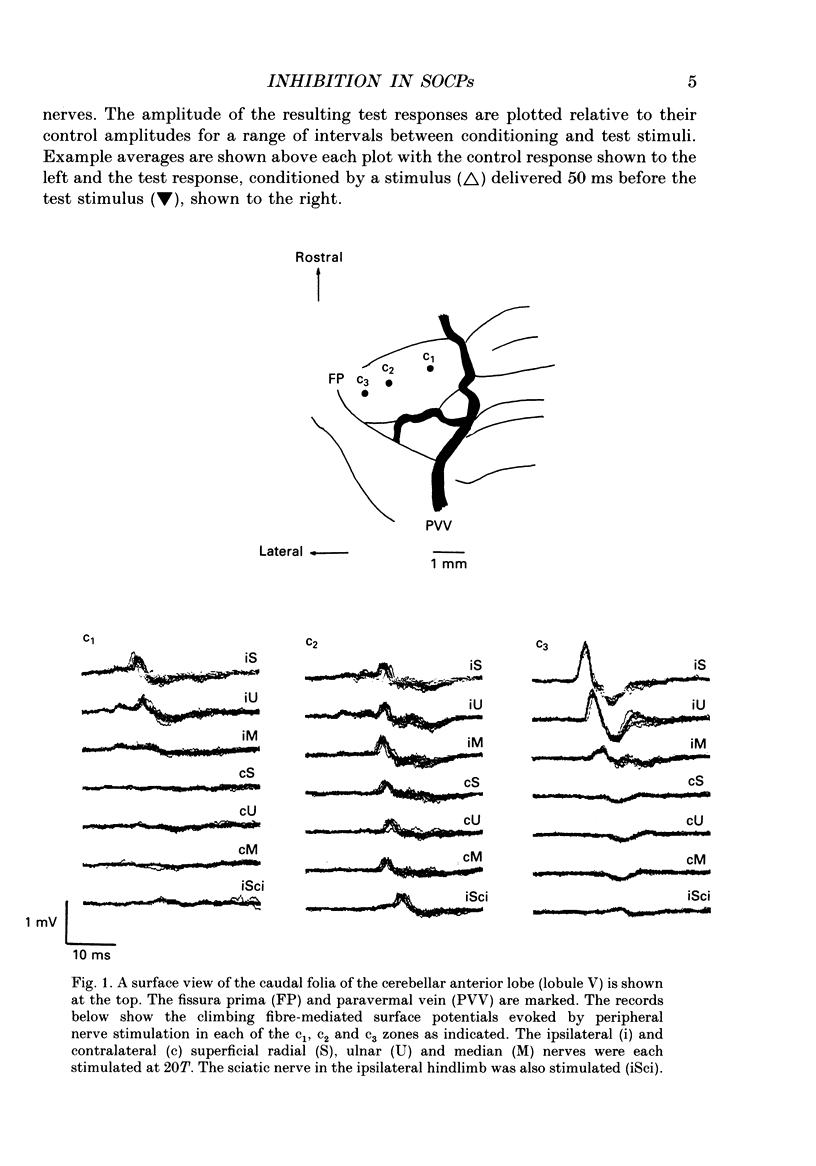
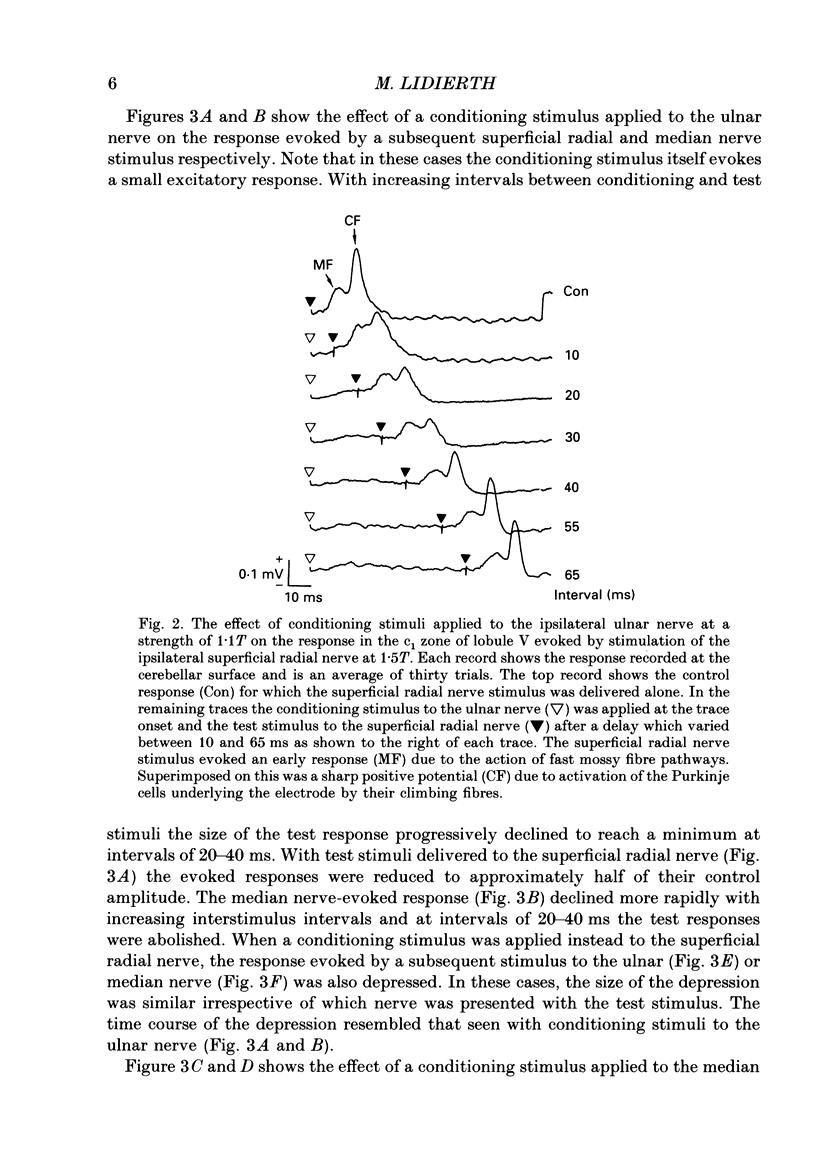
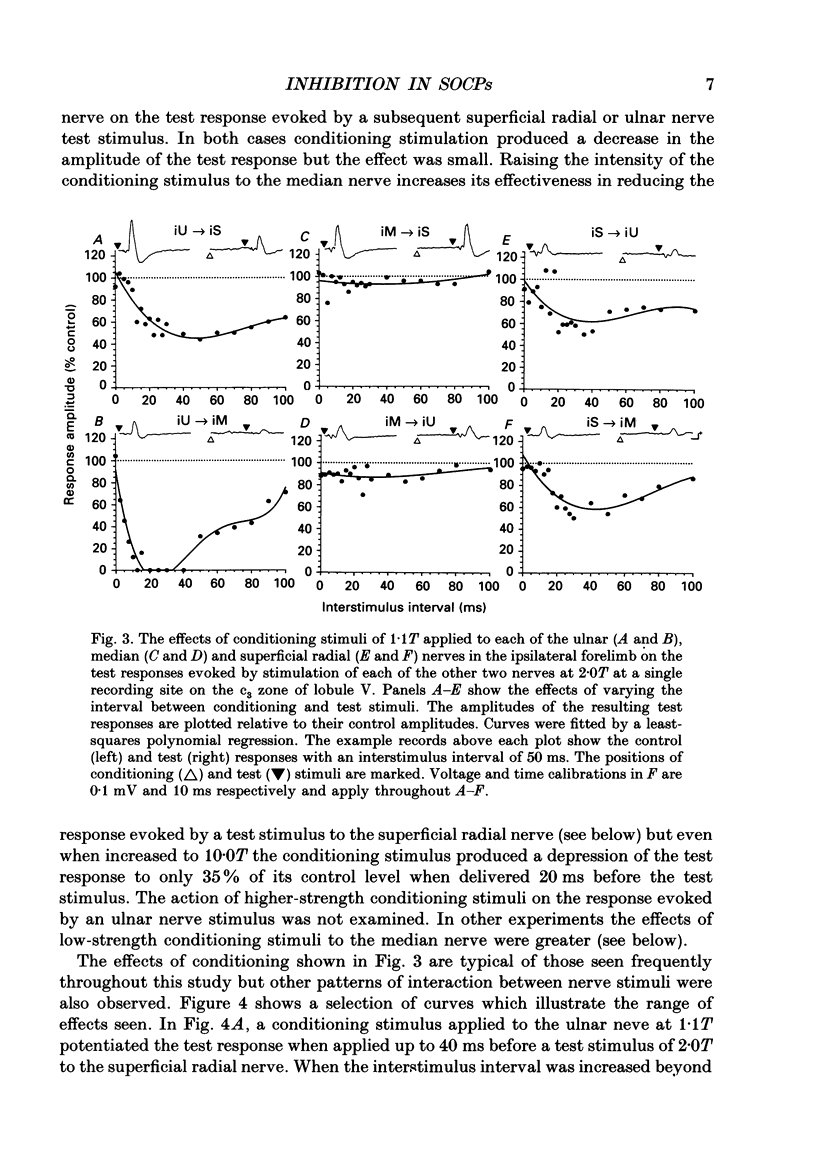
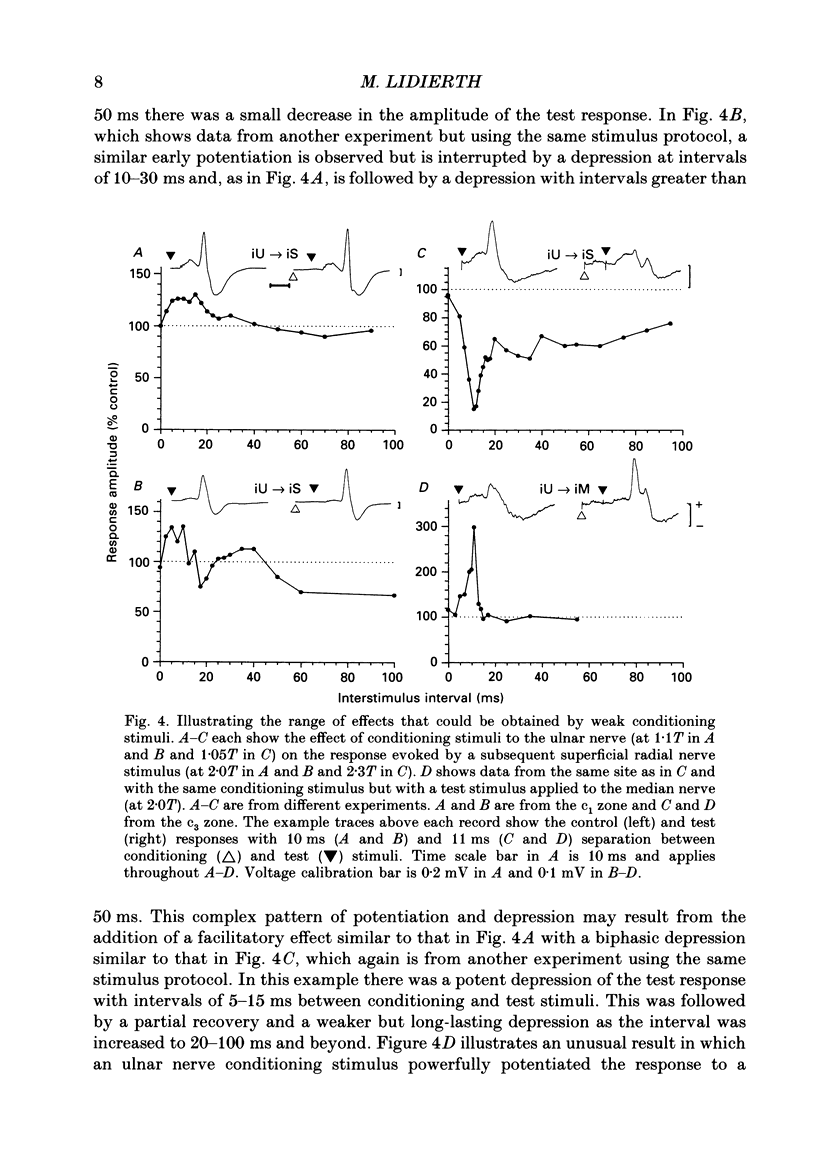
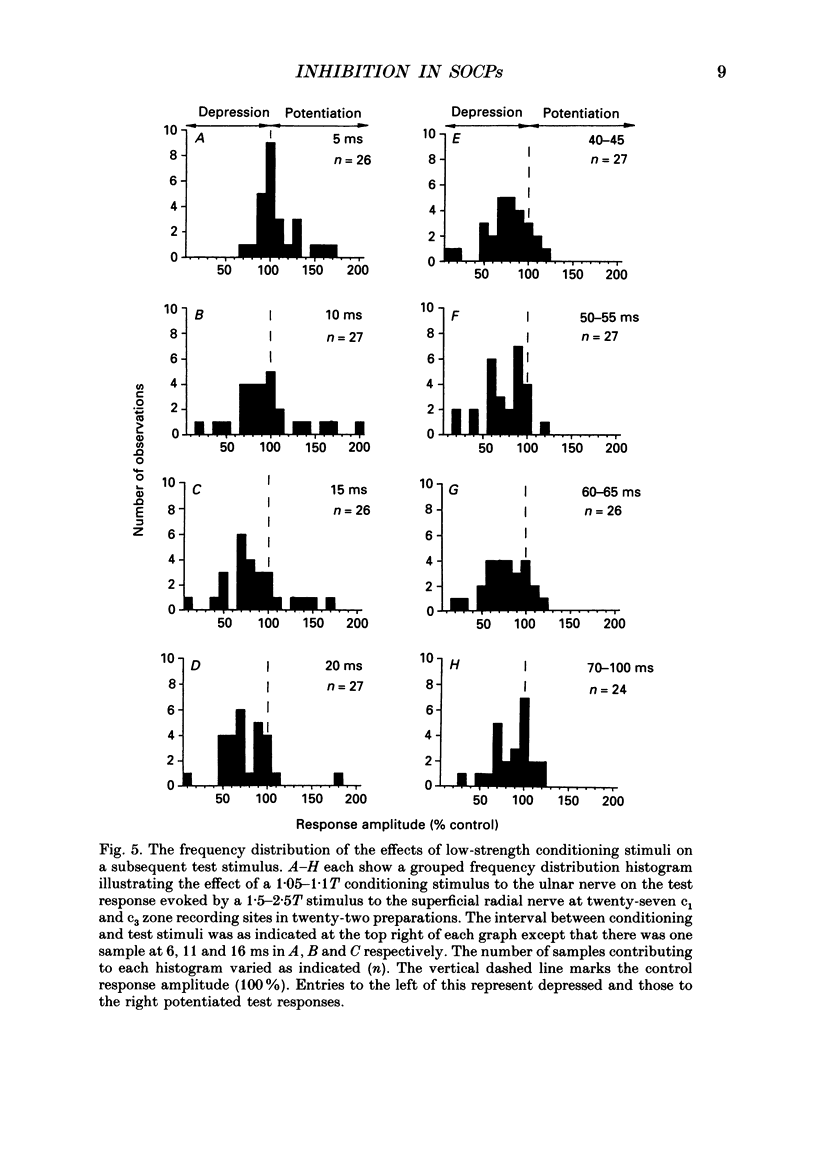
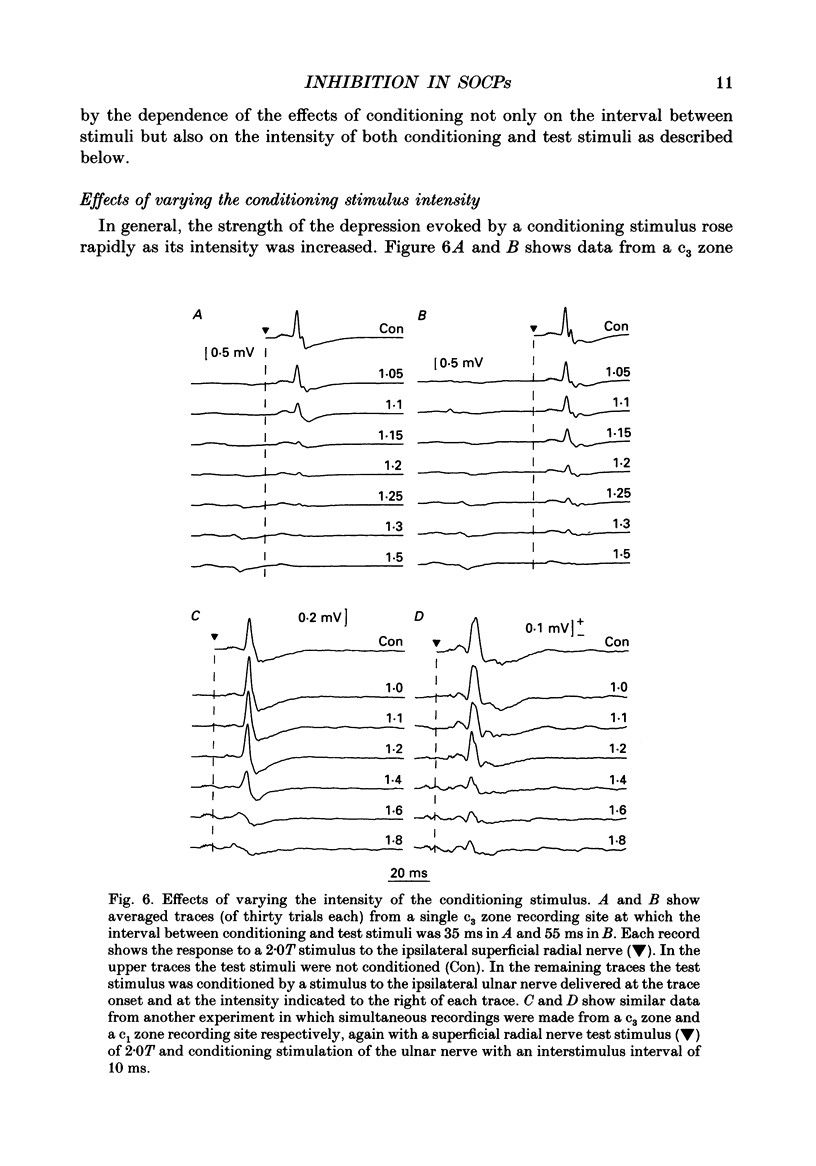
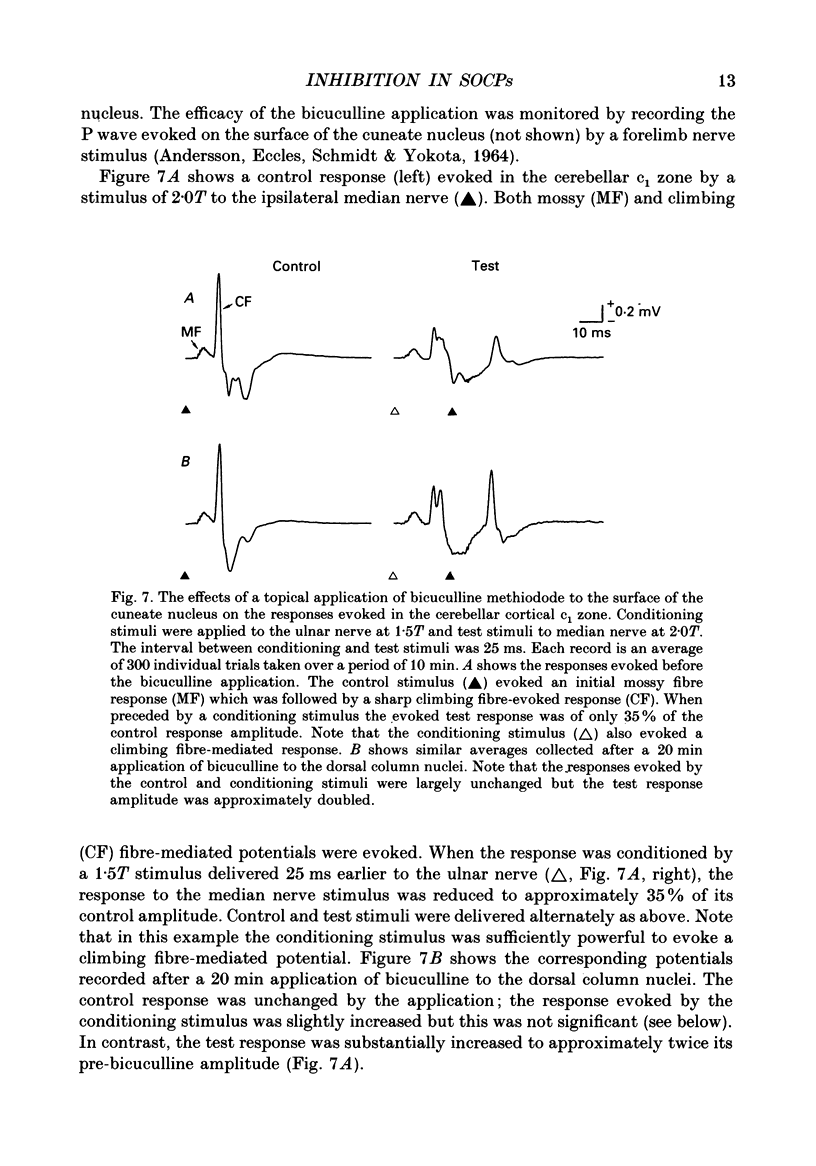
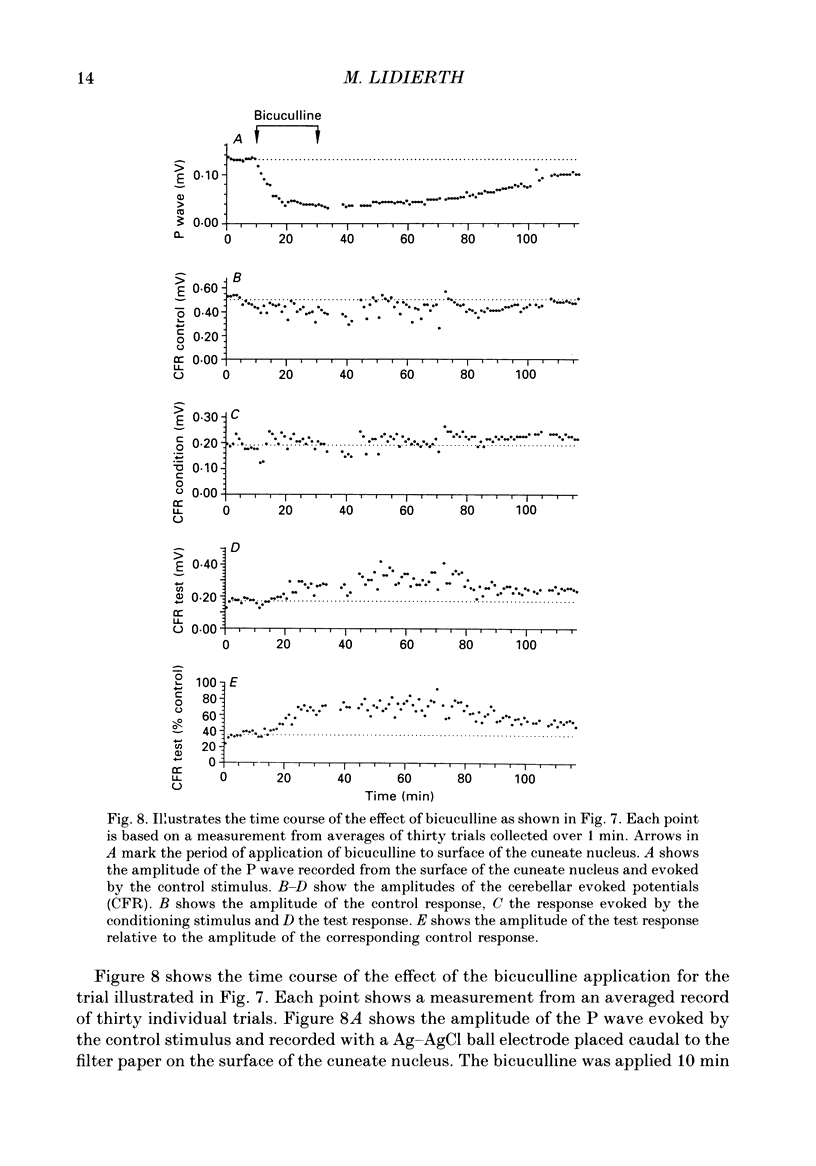
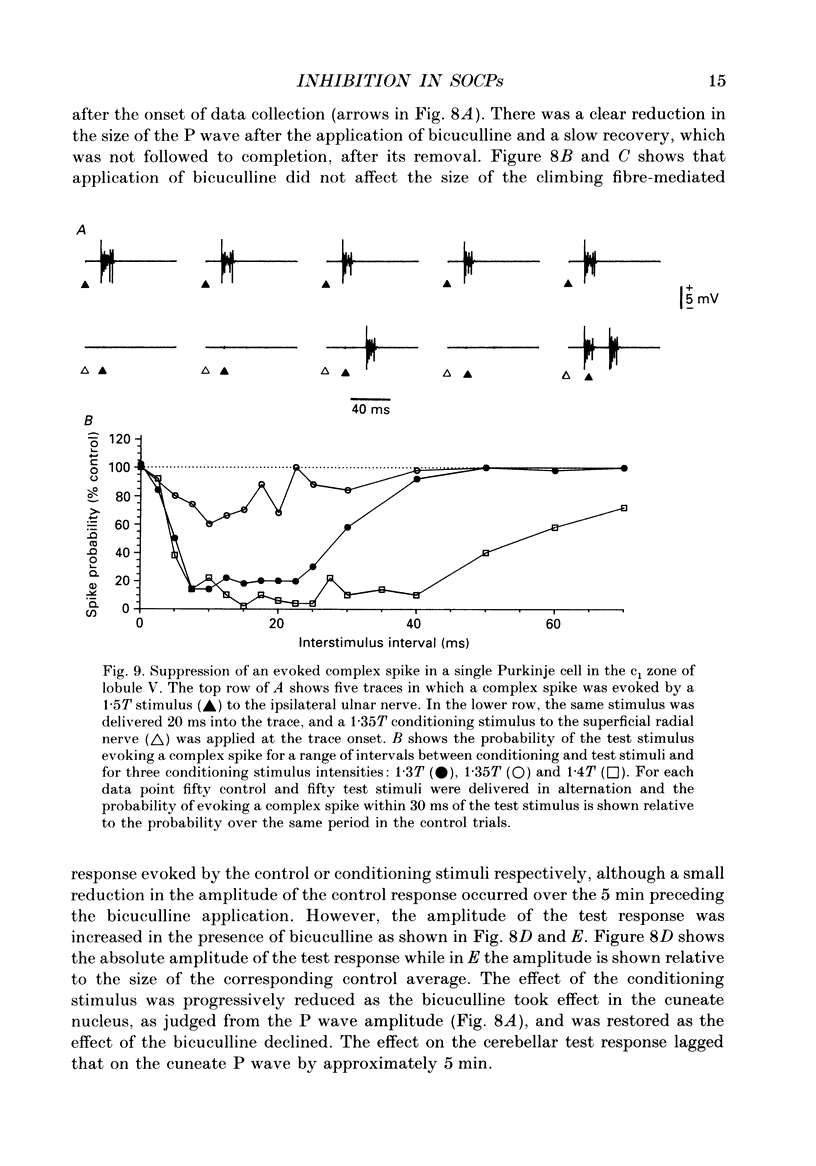
1. The responses evoked by peripheral nerve stimulation in the c1 and c3 zones of the cerebellar cortex have been examined in barbiturate-anaesthetized cats. The responses evoked via the spino-olivocerebellar pathways (SOCPs), which terminate in the cortex as climbing fibres, were recorded as positive multiunit field potentials from the cerebellar surface of lobule V. 2. Low-strength conditioning stimulation of the superficial radial, ulnar or median nerve frequently modified the climbing fibre-mediated responses evoked by a subsequent test stimulus to one of the other nerves. In most cases this modification involved a depression of the evoked response. The depression was not dependent on the conditioning stimulus evoking climbing fibre-mediated responses in the cortex. 3. The depression of the evoked responses increased as the conditioning stimulus intensity was raised within the range of 1.1-1.5 x threshold (1.1-1.5T). 4. Topical application of bicuculline to the surface of the dorsal column nuclei reduced the depression evoked by conditioning stimulation and it is therefore concluded that GABAergic inhibition in the cuneate nucleus contributes to the depression. 5. The inhibition is discussed in relation to its possible contribution to movement-related regulation of the excitability of SOCPs which occurs during locomotion in awake cats.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., ECCLES J. C., OSHIMA T., SCHMIDT R. F. MECHANISMS OF SYNAPTIC TRANSMISSION IN THE CUNEATE NUCLEUS. J Neurophysiol. 1964 Nov;27:1096–1116. doi: 10.1152/jn.1964.27.6.1096. [DOI] [PubMed] [Google Scholar]
- ANDERSEN P., ECCLES J. C., SCHMIDT R. F., YOKOTA T. SLOW POTENTIAL WAVES PRODUCED IN THE CUNEATE NUCLEUS BY CUTANEOUS VOLLEYS AND BY CORTICAL STIMULATION. J Neurophysiol. 1964 Jan;27:78–91. doi: 10.1152/jn.1964.27.1.78. [DOI] [PubMed] [Google Scholar]
- Andersson G., Armstrong D. M. Complex spikes in Purkinje cells in the lateral vermis (b zone) of the cat cerebellum during locomotion. J Physiol. 1987 Apr;385:107–134. doi: 10.1113/jphysiol.1987.sp016487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson G., Garwicz M., Hesslow G. Evidence for a GABA-mediated cerebellar inhibition of the inferior olive in the cat. Exp Brain Res. 1988;72(3):450–456. doi: 10.1007/BF00250590. [DOI] [PubMed] [Google Scholar]
- Andersson G. Mutual inhibition between olivary cell groups projecting to different cerebellar microzones in the cat. Exp Brain Res. 1984;54(2):293–303. doi: 10.1007/BF00236230. [DOI] [PubMed] [Google Scholar]
- Apps R., Lidierth M., Armstrong D. M. Locomotion-related variations in excitability of spino-olivocerebellar paths to cat cerebellar cortical c2 zone. J Physiol. 1990 May;424:487–512. doi: 10.1113/jphysiol.1990.sp018079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong B. D., Harvey R. J. Responses in the inferior olive to stimulation of the cerebellar and cerebral cortices in the cat. J Physiol. 1966 Dec;187(3):553–574. doi: 10.1113/jphysiol.1966.sp008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Eccles J. C., Harvey R. J., Matthews P. B. Responses in the dorsal accessory olive of the cat to stimulation of hind limb afferents. J Physiol. 1968 Jan;194(1):125–145. doi: 10.1113/jphysiol.1968.sp008398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Edgley S. A., Lidierth M. Complex spikes in Purkinje cells of the paravermal part of the anterior lobe of the cat cerebellum during locomotion. J Physiol. 1988 Jun;400:405–414. doi: 10.1113/jphysiol.1988.sp017128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Harvey R. J. Responses to a spino-olivo-cerebellar pathway in the cat. J Physiol. 1968 Jan;194(1):147–168. doi: 10.1113/jphysiol.1968.sp008399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A. Bicuculline, an antagonist of GABA and synaptic inhibition in the spinal cord of the cat. Brain Res. 1971 Sep 10;32(1):69–96. doi: 10.1016/0006-8993(71)90156-9. [DOI] [PubMed] [Google Scholar]
- Ekerot C. F., Larson B. The dorsal spino-olivocerebellar system in the cat. I. Functional organization and termination in the anterior lobe. Exp Brain Res. 1979 Jul 2;36(2):201–217. doi: 10.1007/BF00238905. [DOI] [PubMed] [Google Scholar]
- Gellman R., Gibson A. R., Houk J. C. Inferior olivary neurons in the awake cat: detection of contact and passive body displacement. J Neurophysiol. 1985 Jul;54(1):40–60. doi: 10.1152/jn.1985.54.1.40. [DOI] [PubMed] [Google Scholar]
- Gellman R., Houk J. C., Gibson A. R. Somatosensory properties of the inferior olive of the cat. J Comp Neurol. 1983 Apr 1;215(2):228–243. doi: 10.1002/cne.902150210. [DOI] [PubMed] [Google Scholar]
- Groenewegen H. J., Voogd J., Freedman S. L. The parasagittal zonation within the olivocerebellar projection. II. Climbing fiber distribution in the intermediate and hemispheric parts of cat cerebellum. J Comp Neurol. 1979 Feb 1;183(3):551–601. doi: 10.1002/cne.901830307. [DOI] [PubMed] [Google Scholar]
- Larson B., Miller S., Oscarsson O. A spinocerebellar climbing fibre path activated by the flexor reflex afferents from all four limbs. J Physiol. 1969 Aug;203(3):641–649. doi: 10.1113/jphysiol.1969.sp008883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson B., Miller S., Oscarsson O. Termination and functional organization of the dorsolateral spino-olivocerebellar path. J Physiol. 1969 Aug;203(3):611–640. doi: 10.1113/jphysiol.1969.sp008882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leicht R., Rowe M. J., Schmidt R. F. Cortical and peripheral modification of cerebellar climbing fibre activity arising from cutaneous mechanoreceptors. J Physiol. 1973 Feb;228(3):619–635. doi: 10.1113/jphysiol.1973.sp010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidierth M., Apps R. Gating in the spino-olivocerebellar pathways to the c1 zone of the cerebellar cortex during locomotion in the cat. J Physiol. 1990 Nov;430:453–469. doi: 10.1113/jphysiol.1990.sp018301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman P. P., Paul D. H. The effects of stimulating cutaneous and splanchnic afferents on cerebellar unit discharges. J Physiol. 1966 Dec;187(3):575–582. doi: 10.1113/jphysiol.1966.sp008109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscarsson O. Termination and functional organization of the dorsal spino-olivocerebellar path. J Physiol. 1969 Jan;200(1):129–149. doi: 10.1113/jphysiol.1969.sp008685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda T., Maekawa K. Bilateral visual inputs to the dorsal cap of inferior olive: differential localization and inhibitory interactions. Exp Brain Res. 1980;39(4):461–471. doi: 10.1007/BF00239311. [DOI] [PubMed] [Google Scholar]


