Abstract
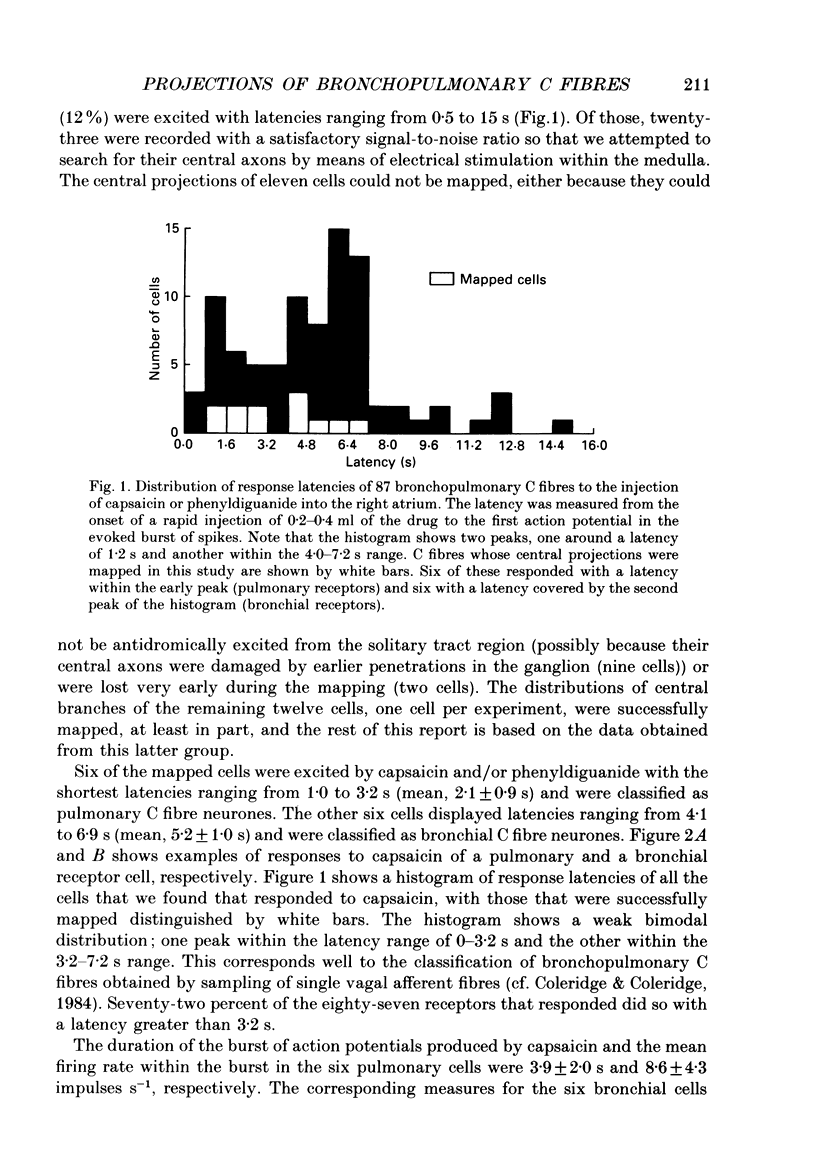
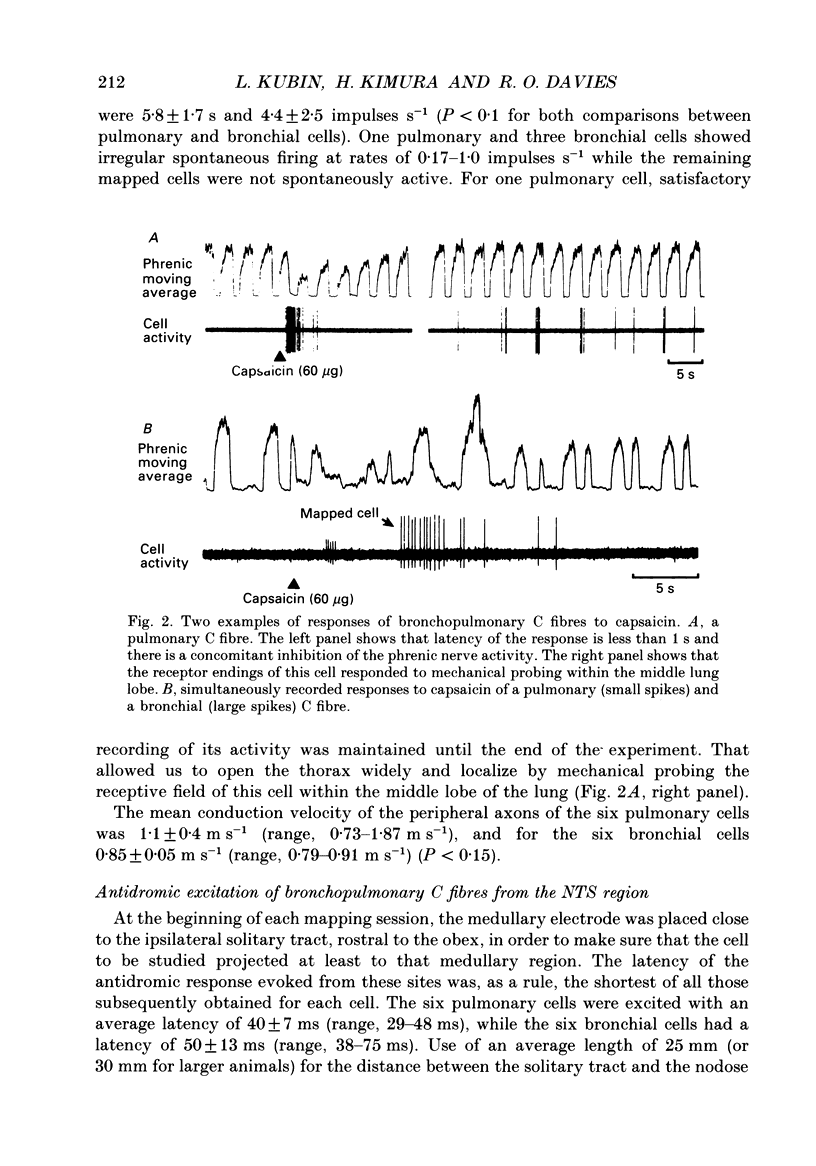
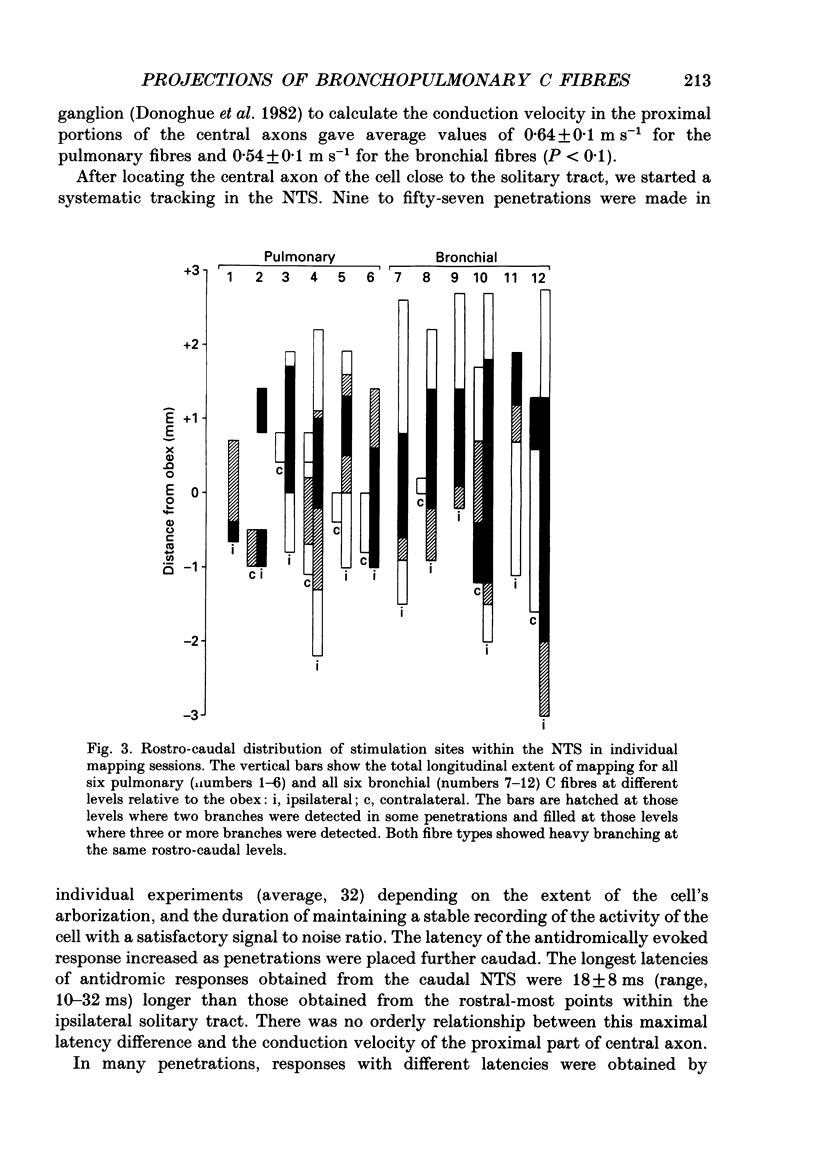
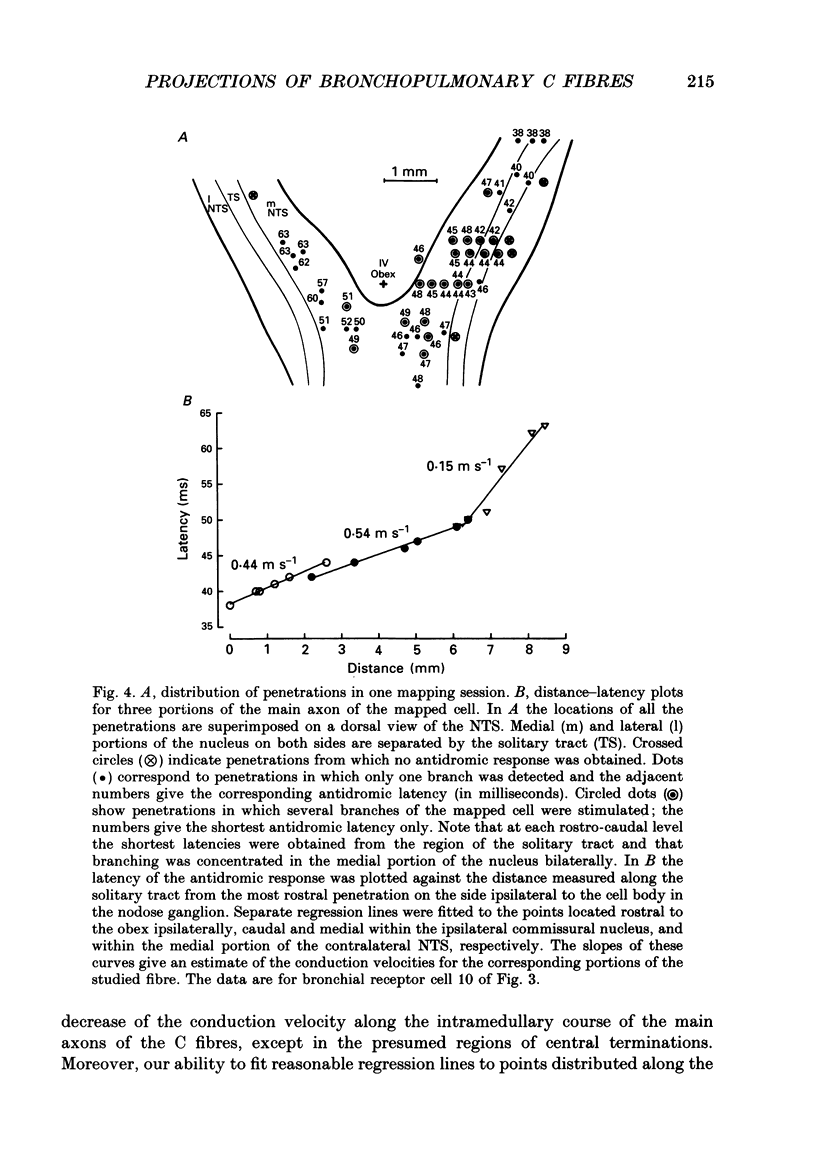
1. The activity of eighty-seven bronchopulmonary vagal afferent neurones with unmyelinated axons (C fibres) was recorded extracellularly in the nodose ganglia of decerebrate, paralysed and artificially ventilated cats. On the basis of their response latencies following the right atrial injection of capsaicin or phenyldiguanide, the cells were classified as having their receptor endings within the reach of pulmonary (latency less than 3.5 s) or bronchial (latency above 3.5 s) circulation. 2. Pulmonary and bronchial receptor cells differed only slightly in their response characteristics (firing rate, burst duration) and the conduction velocity of their peripheral axons. Bronchial C fibres represented about 70% of the population studied. 3. The medullary distributions of the central branches of six pulmonary and six bronchial C fibres were determined by means of the antidromic mapping technique. The two receptor subtypes did not differ in their central projection patterns. 4. Rostral to the obex, the central branches of the bronchopulmonary C fibres were localized within the medial portions of the nucleus tractus solitarii (NTS) and area postrema, and were most densely distributed along the borders of the parvicellular subnucleus of the NTS. Caudal to the obex, the most dense branching was found in the dorsal portion of the commissural subnucleus. Projections to the contralateral NTS were found, but these were of a much lower density. 5. The central distribution of bronchopulmonary C fibres is compared to the projection patterns of vagal and glossopharyngeal afferents of other modalities that are involved in respiratory and cardiovascular control. This is discussed in relation to the concept of a modality-specific organization of the NTS.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AGOSTONI E., CHINNOCK J. E., DE DALY M. B., MURRAY J. G. Functional and histological studies of the vagus nerve and its branches to the heart, lungs and abdominal viscera in the cat. J Physiol. 1957 Jan 23;135(1):182–205. doi: 10.1113/jphysiol.1957.sp005703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baude A., Lanoir J., Vernier P., Puizillout J. J. Substance P-immunoreactivity in the dorsal medial region of the medulla in the cat: effects of nodosectomy. J Chem Neuroanat. 1989 Mar-Apr;2(2):67–81. [PubMed] [Google Scholar]
- Bennett J. A., Goodchild C. S., Kidd C., McWilliam P. N. Neurones in the brain stem of the cat excited by vagal afferent fibres from the heart and lungs. J Physiol. 1985 Dec;369:1–15. doi: 10.1113/jphysiol.1985.sp015884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A. J., Averill D. B. Projection of single pulmonary stretch receptors to solitary tract region. J Neurophysiol. 1983 Mar;49(3):819–830. doi: 10.1152/jn.1983.49.3.819. [DOI] [PubMed] [Google Scholar]
- Bras H., Cavallari P., Jankowska E., Kubin L. Morphology of midlumbar interneurones relaying information from group II muscle afferents in the cat spinal cord. J Comp Neurol. 1989 Dec 1;290(1):1–15. doi: 10.1002/cne.902900102. [DOI] [PubMed] [Google Scholar]
- COTTLE M. K. DEGENERATION STUDIES OF PRIMARY AFFERENTS OF IXTH AND XTH CRANIAL NERVES IN THE CAT. J Comp Neurol. 1964 Jun;122:329–345. doi: 10.1002/cne.901220304. [DOI] [PubMed] [Google Scholar]
- Claps A., Torrealba F. The carotid body connections: a WGA-HRP study in the cat. Brain Res. 1988 Jul 5;455(1):123–133. doi: 10.1016/0006-8993(88)90121-7. [DOI] [PubMed] [Google Scholar]
- Coleridge J. C., Coleridge H. M. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol. 1984;99:1–110. doi: 10.1007/BFb0027715. [DOI] [PubMed] [Google Scholar]
- Czachurski J., Dembowsky K., Seller H., Nobiling R., Taugner R. Morphology of electrophysiologically identified baroreceptor afferents and second order neurones in the brainstem of the cat. Arch Ital Biol. 1988 Jun;126(3):129–144. [PubMed] [Google Scholar]
- Daly M. D., Kirkman E. Cardiovascular responses to stimulation of pulmonary C fibres in the cat: their modulation by changes in respiration. J Physiol. 1988 Aug;402:43–63. doi: 10.1113/jphysiol.1988.sp017193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. O., Kubin L., Pack A. I. Pulmonary stretch receptor relay neurones of the cat: location and contralateral medullary projections. J Physiol. 1987 Feb;383:571–585. doi: 10.1113/jphysiol.1987.sp016429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. O., Kubin L. Projection of pulmonary rapidly adapting receptors to the medulla of the cat: an antidromic mapping study. J Physiol. 1986 Apr;373:63–86. doi: 10.1113/jphysiol.1986.sp016035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpierre S., Grimaud C., Jammes Y., Mei N. Changes in activity of vagal bronchopulmonary C fibres by chemical and physical stimuli in the cat. J Physiol. 1981 Jul;316:61–74. doi: 10.1113/jphysiol.1981.sp013772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue S., Felder R. B., Jordan D., Spyer K. M. The central projections of carotid baroreceptors and chemoreceptors in the cat: a neurophysiological study. J Physiol. 1984 Feb;347:397–409. doi: 10.1113/jphysiol.1984.sp015072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue S., Garcia M., Jordan D., Spyer K. M. The brain-stem projections of pulmonary stretch afferent neurones in cats and rabbits. J Physiol. 1982 Jan;322:353–363. doi: 10.1113/jphysiol.1982.sp014041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka N., Mannen H., Hongo T., Sasaki S. Trajectory of group Ia afferent fibers stained with horseradish peroxidase in the lumbosacral spinal cord of the cat: three dimensional reconstructions from serial sections. J Comp Neurol. 1979 Jul 15;186(2):189–211. doi: 10.1002/cne.901860206. [DOI] [PubMed] [Google Scholar]
- Jammes Y., Fornaris E., Mei N., Barrat E. Afferent and efferent components of the bronchial vagal branches in cats. J Auton Nerv Syst. 1982 Mar;5(2):165–176. doi: 10.1016/0165-1838(82)90037-6. [DOI] [PubMed] [Google Scholar]
- KERR F. W. Facial, vagal and glossopharyngeal nerves in the cat. Afferent connections. Arch Neurol. 1962 Apr;6:264–281. doi: 10.1001/archneur.1962.00450220006003. [DOI] [PubMed] [Google Scholar]
- Kalia M., Mesulam M. M. Brain stem projections of sensory and motor components of the vagus complex in the cat: I. The cervical vagus and nodose ganglion. J Comp Neurol. 1980 Sep 15;193(2):435–465. doi: 10.1002/cne.901930210. [DOI] [PubMed] [Google Scholar]
- Kalia M., Mesulam M. M. Brain stem projections of sensory and motor components of the vagus complex in the cat: II. Laryngeal, tracheobronchial, pulmonary, cardiac, and gastrointestinal branches. J Comp Neurol. 1980 Sep 15;193(2):467–508. doi: 10.1002/cne.901930211. [DOI] [PubMed] [Google Scholar]
- Kalia M., Richter D. Morphology of physiologically identified slowly adapting lung stretch receptor afferents stained with intra-axonal horseradish peroxidase in the nucleus of the tractus solitarius of the cat. I. A light microscopic analysis. J Comp Neurol. 1985 Nov 22;241(4):503–520. doi: 10.1002/cne.902410409. [DOI] [PubMed] [Google Scholar]
- Kalia M., Richter D. Rapidly adapting pulmonary receptor afferents: I. Arborization in the nucleus of the tractus solitarius. J Comp Neurol. 1988 Aug 22;274(4):560–573. doi: 10.1002/cne.902740406. [DOI] [PubMed] [Google Scholar]
- Lipski J. Antidromic activation of neurones as an analytic tool in the study of the central nervous system. J Neurosci Methods. 1981 Jun;4(1):1–32. doi: 10.1016/0165-0270(81)90015-7. [DOI] [PubMed] [Google Scholar]
- Loewy A. D., Burton H. Nuclei of the solitary tract: efferent projections to the lower brain stem and spinal cord of the cat. J Comp Neurol. 1978 Sep 15;181(2):421–449. doi: 10.1002/cne.901810211. [DOI] [PubMed] [Google Scholar]
- Maley B. E., Sasek C. A., Seybold V. S. Substance P binding sites in the nucleus tractus solitarius of the cat. Peptides. 1988 Nov-Dec;9(6):1301–1306. doi: 10.1016/0196-9781(88)90196-9. [DOI] [PubMed] [Google Scholar]
- Maley B., Elde R. Immunohistochemical localization of putative neurotransmitters within the feline nucleus tractus solitarii. Neuroscience. 1982 Oct;7(10):2469–2490. doi: 10.1016/0306-4522(82)90208-1. [DOI] [PubMed] [Google Scholar]
- Mei N., Condamin M., Boyer A. The composition of the vagus nerve of the cat. Cell Tissue Res. 1980;209(3):423–431. doi: 10.1007/BF00234756. [DOI] [PubMed] [Google Scholar]
- Nomura S., Mizuno N. Central distribution of efferent and afferent components of the cervical branches of the vagus nerve. A HRP study in the cat. Anat Embryol (Berl) 1983;166(1):1–18. doi: 10.1007/BF00317941. [DOI] [PubMed] [Google Scholar]
- Paintal A. S. Vagal sensory receptors and their reflex effects. Physiol Rev. 1973 Jan;53(1):159–227. doi: 10.1152/physrev.1973.53.1.159. [DOI] [PubMed] [Google Scholar]
- Russell L. C., Burchiel K. J. Neurophysiological effects of capsaicin. Brain Res. 1984 Dec;320(2-3):165–176. doi: 10.1016/0165-0173(84)90005-5. [DOI] [PubMed] [Google Scholar]
- Sugiura Y., Lee C. L., Perl E. R. Central projections of identified, unmyelinated (C) afferent fibers innervating mammalian skin. Science. 1986 Oct 17;234(4774):358–361. doi: 10.1126/science.3764416. [DOI] [PubMed] [Google Scholar]
- Torrealba F., Calderón F. Central projections of coarse and fine vagal axons of the cat. Brain Res. 1990 Mar 5;510(2):351–354. doi: 10.1016/0006-8993(90)91390-3. [DOI] [PubMed] [Google Scholar]
- Torrealba F., Claps A. The carotid sinus connections: a WGA-HRP study in the cat. Brain Res. 1988 Jul 5;455(1):134–143. doi: 10.1016/0006-8993(88)90122-9. [DOI] [PubMed] [Google Scholar]


