Abstract
1. Excitatory postsynaptic potentials (EPSPs) and their underlying currents (EPSCs) were recorded from dentate granule cells in thin hippocampal slices of rats using the tight-seal whole-cell recording technique. 2. At resting membrane potentials (ca -60 to -70 mV), the EPSCs clearly consisted of a dominant fast and a smaller slow component. The slow EPSC component markedly increased with depolarization. This resulted in a region of negative slope conductance (between -50 and -30 mV) in the peak current-voltage (I-V) relation of the dual-component EPSC in most neurones. The EPSCs reversed entirely at -1.2 +/- 2.8 mV (n = 15). 3. Using selective antagonists of N-methyl-D-aspartate (NMDA) and non-NMDA excitatory amino acid receptors, two pharmacologically distinct components of the natural EPSCs were isolated. The non-NMDA EPSCs displayed a linear I-V relation. Their rise times (0.5-1.9 ms) were independent of membrane voltage but seemed to depend critically on the precise dendritic location of the synapse. Their decay was approximated by a single exponential with a time constant ranging from 3 to 9 ms. The time course of these EPSCs was independent of changes in extracellular Mg2+. 4. The NMDA EPSCs displayed a non-linear I-V relation. At resting membrane potentials their peak amplitudes were 20 pA and increased steadily with depolarization to -30 mV. At membrane voltages positive to -30 mV the peak I-V relation was linear. The rise times of NMDA EPSCs ranged from 4 to 9 ms and were insensitive to membrane voltage. 5. The NMDA EPSCs decayed biexponentially. Both time constants, tau f and tau s, increased with depolarization in an exponential manner, tau s being more voltage dependent than tau f. Lowering extracellular Mg2+ slightly reduced both rate constants but did not completely abolish their voltage sensitivity. 6. Bath application of NMDA to outside-out patches from granule cells induced single channel currents of 52 pS in nominally Mg(2+)-free solutions. They displayed a burst-like single-channel activity with clusters of bursts lasting several hundreds of milliseconds. Currents through single NMDA receptor channels reversed around 0 mV. 7. The fractional contributions of NMDA and non-NMDA components to peak currents and synaptic charge transfer were assessed. At resting membrane potential the NMDA EPSC component accounted for 23% of the peak current and for 64% of the synaptic charge transfer. The contribution of the NMDA EPSC component to the synaptic charge transfer strongly increased with small depolarizations from rest.
Full text
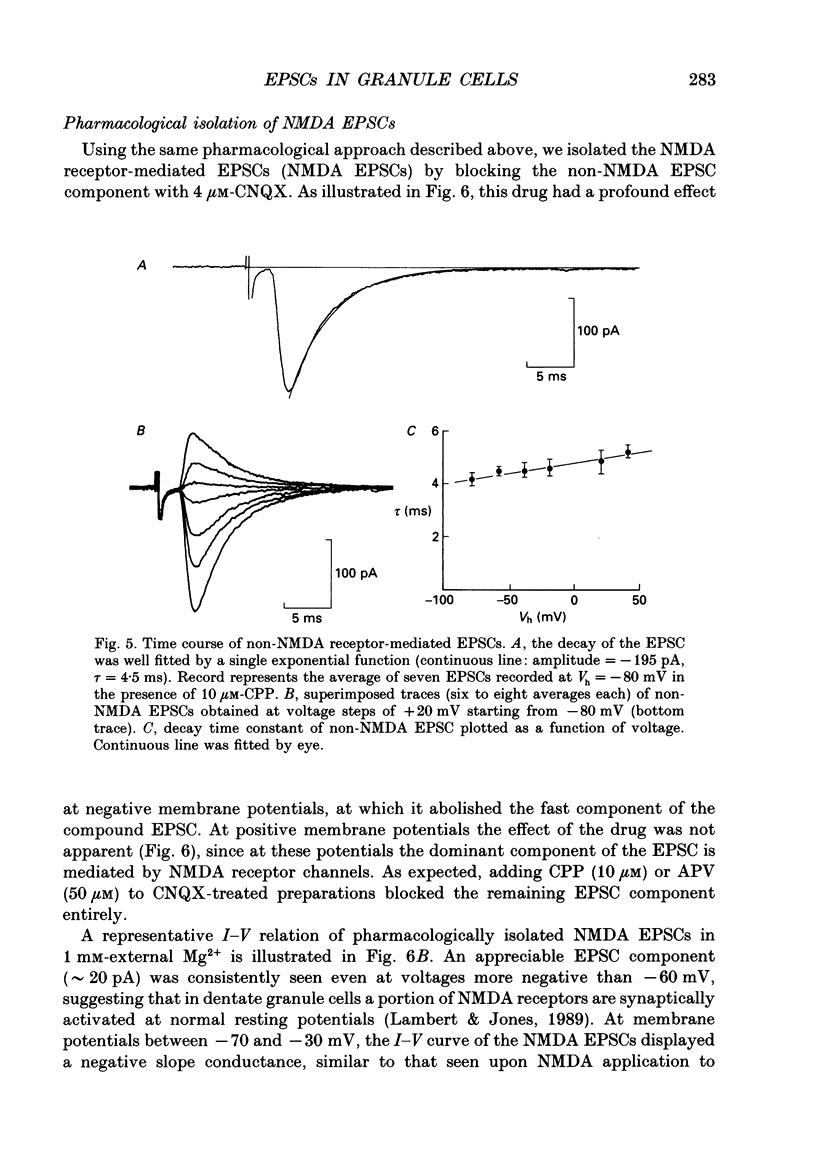
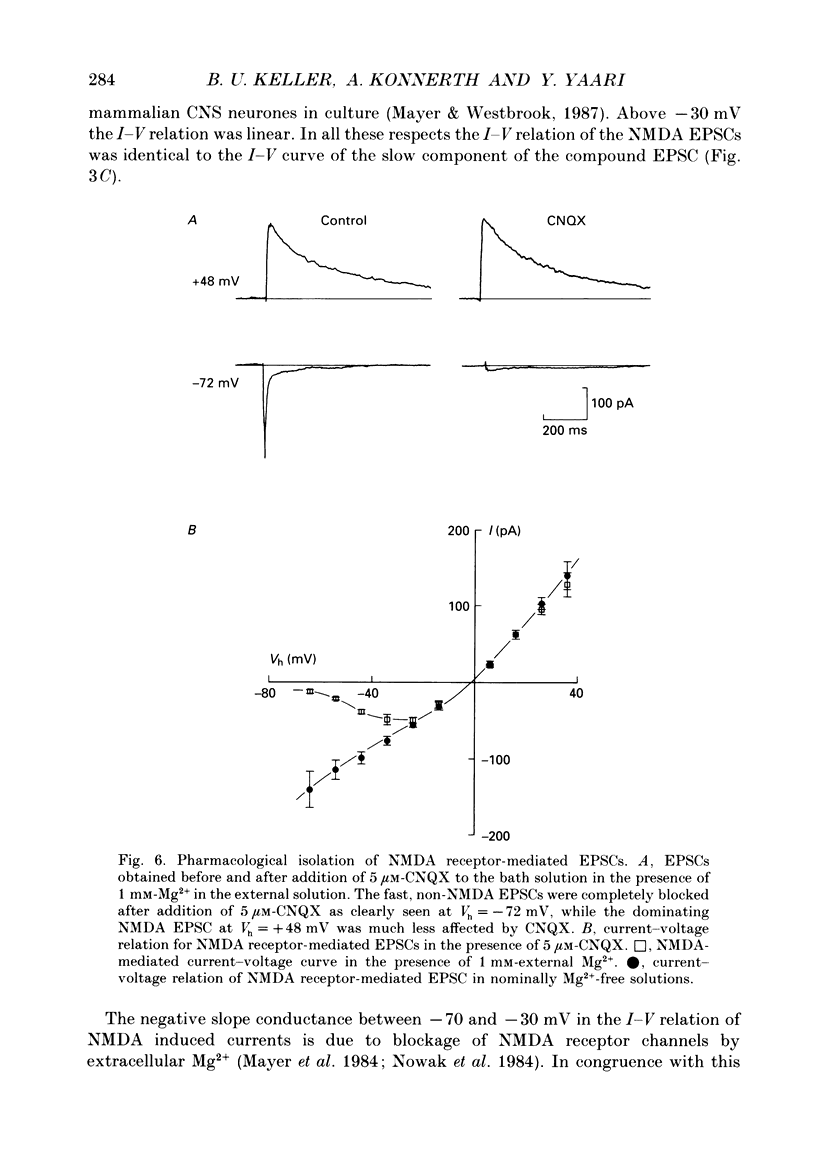
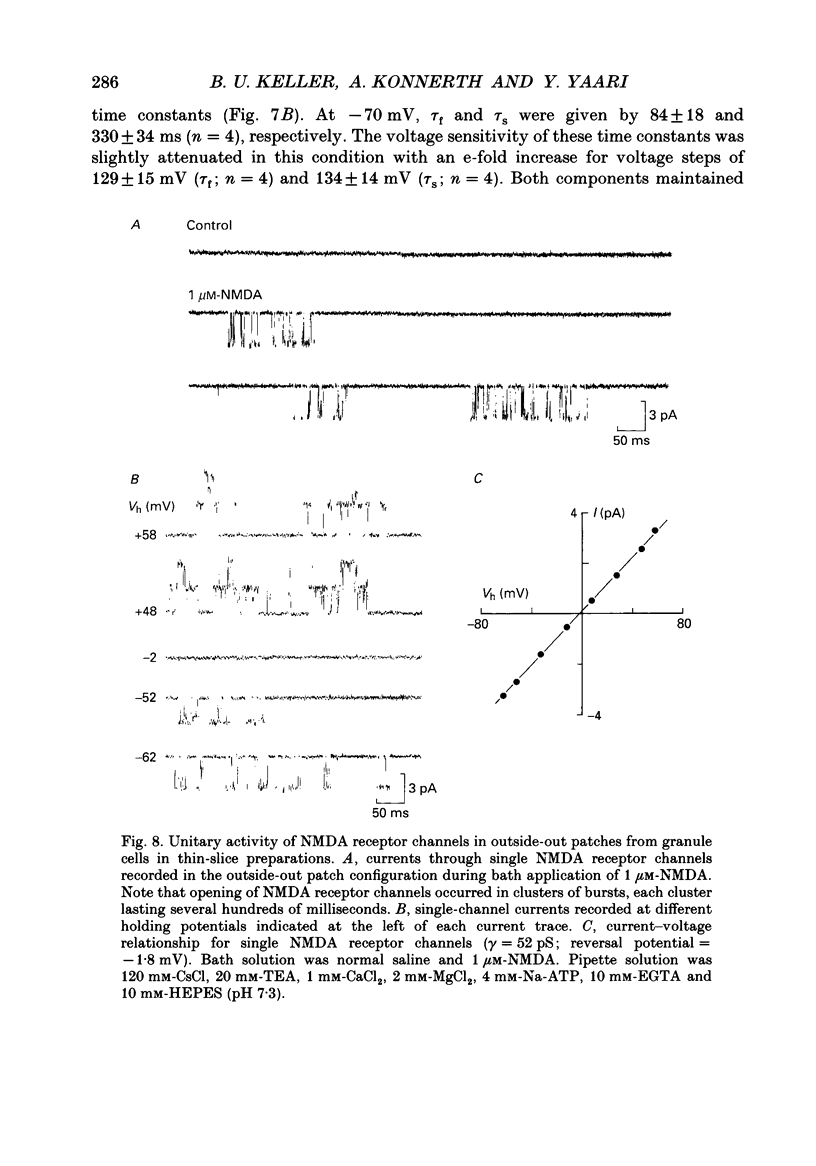
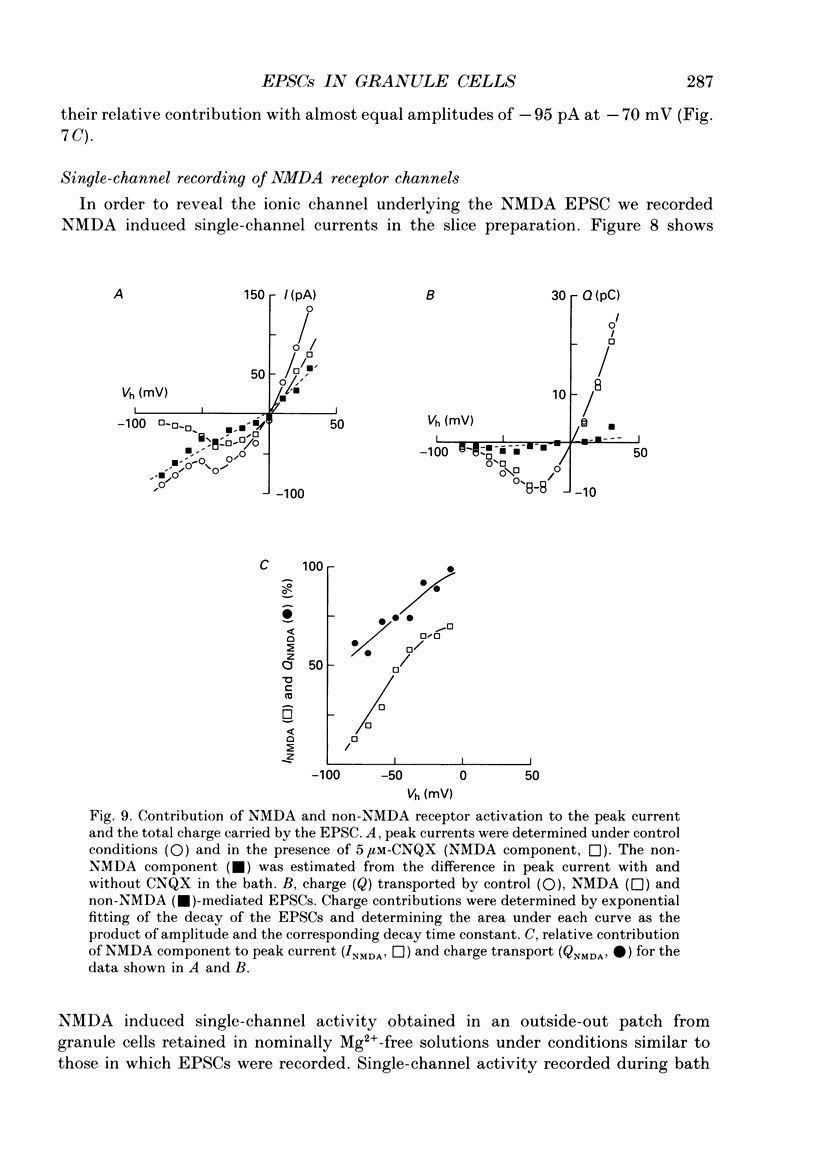
PDF

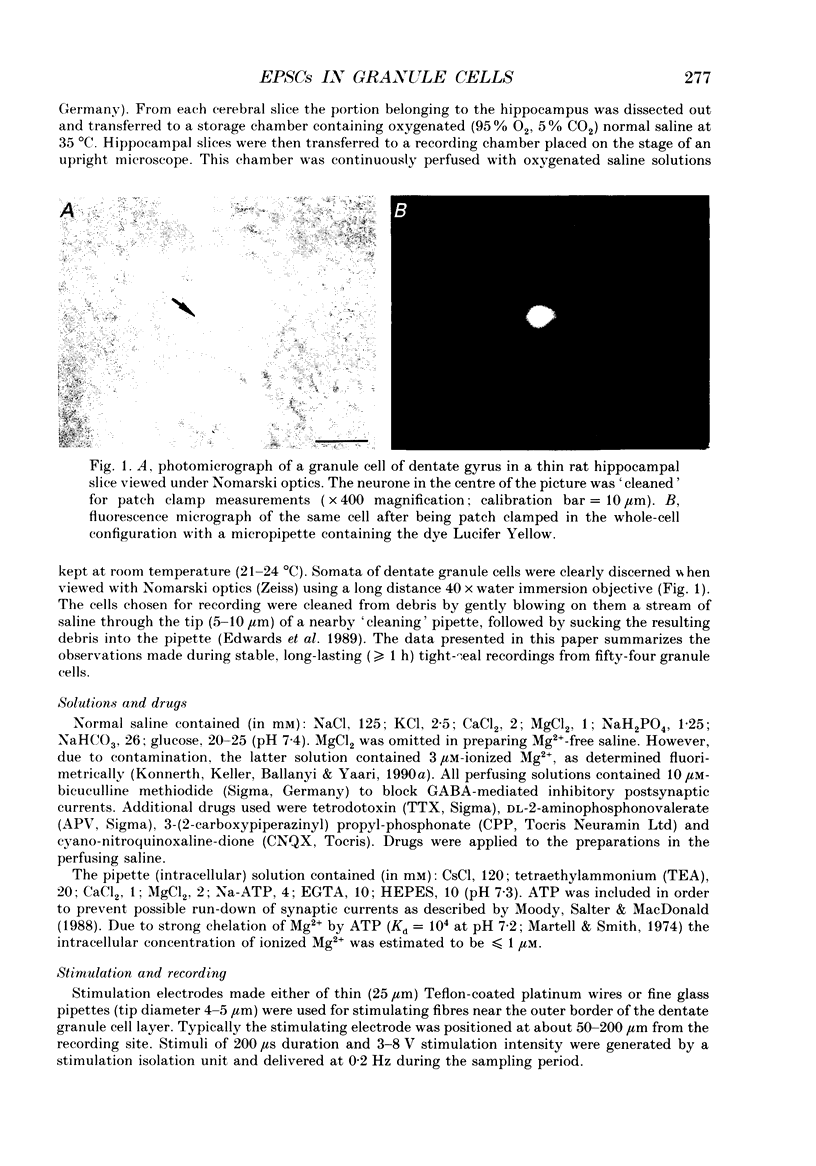

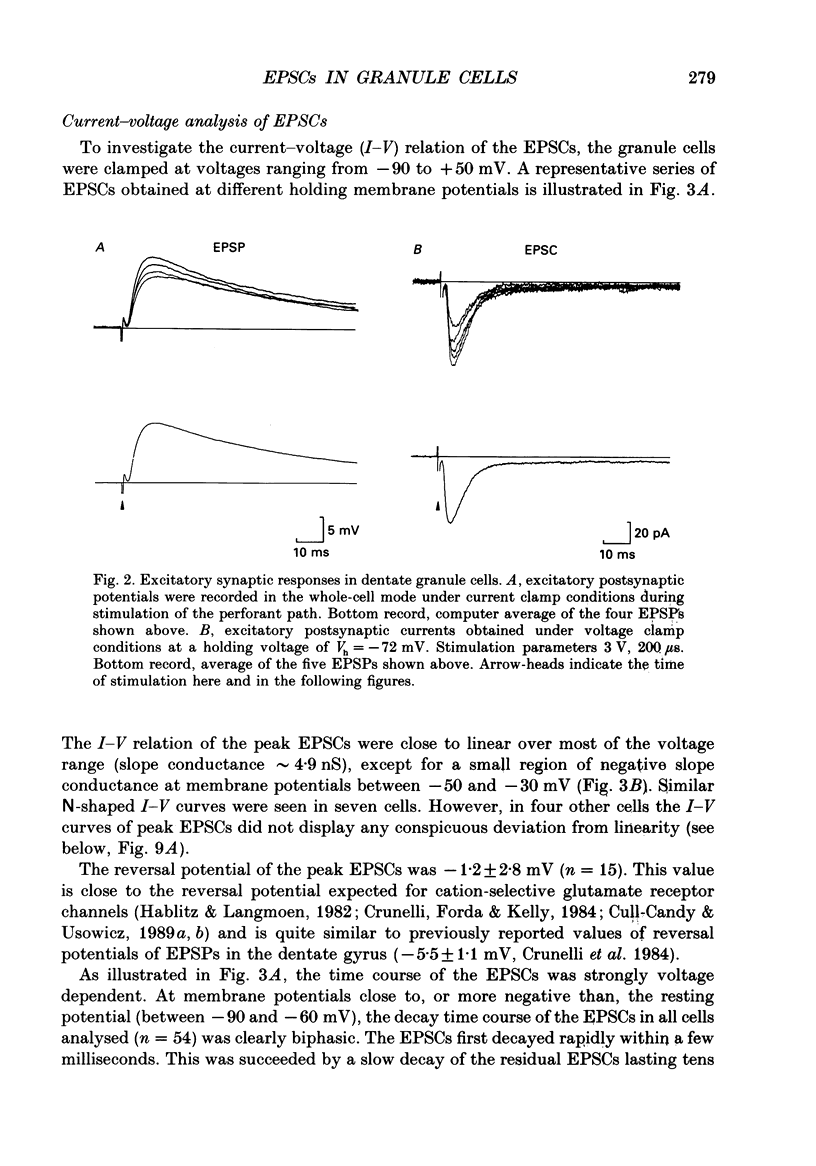
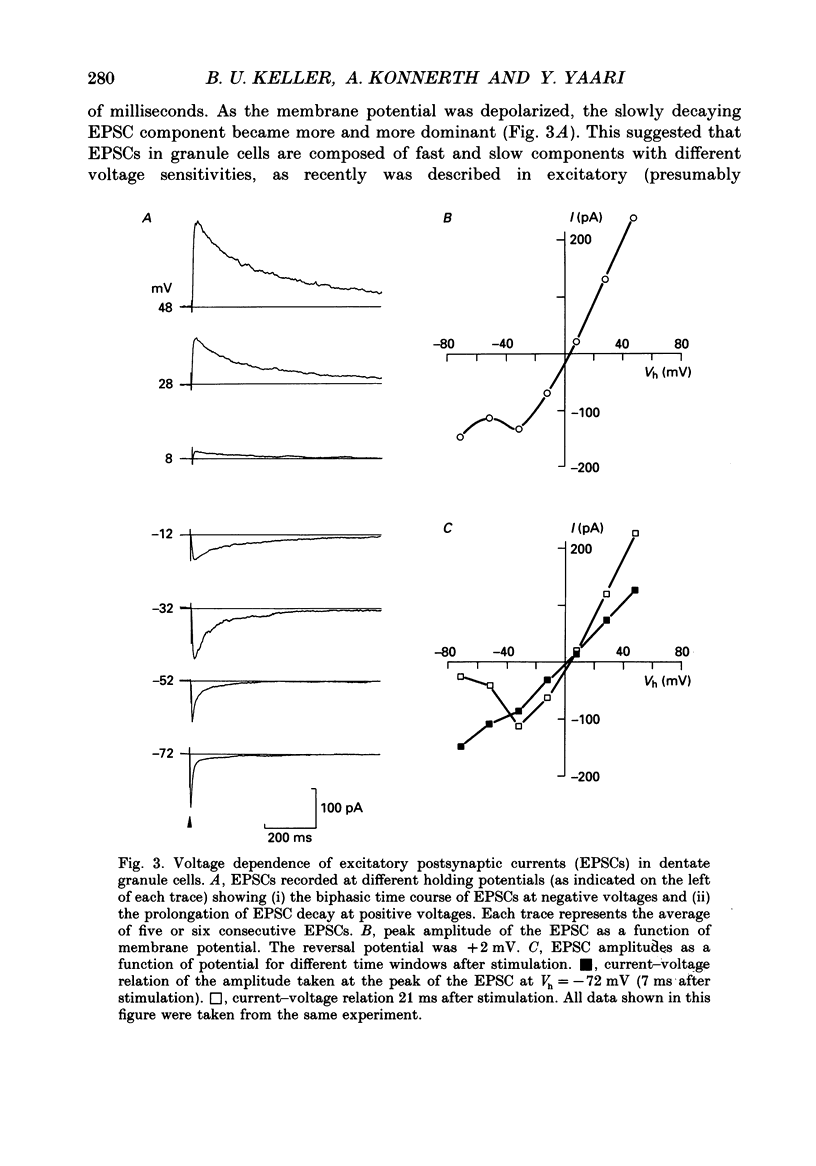

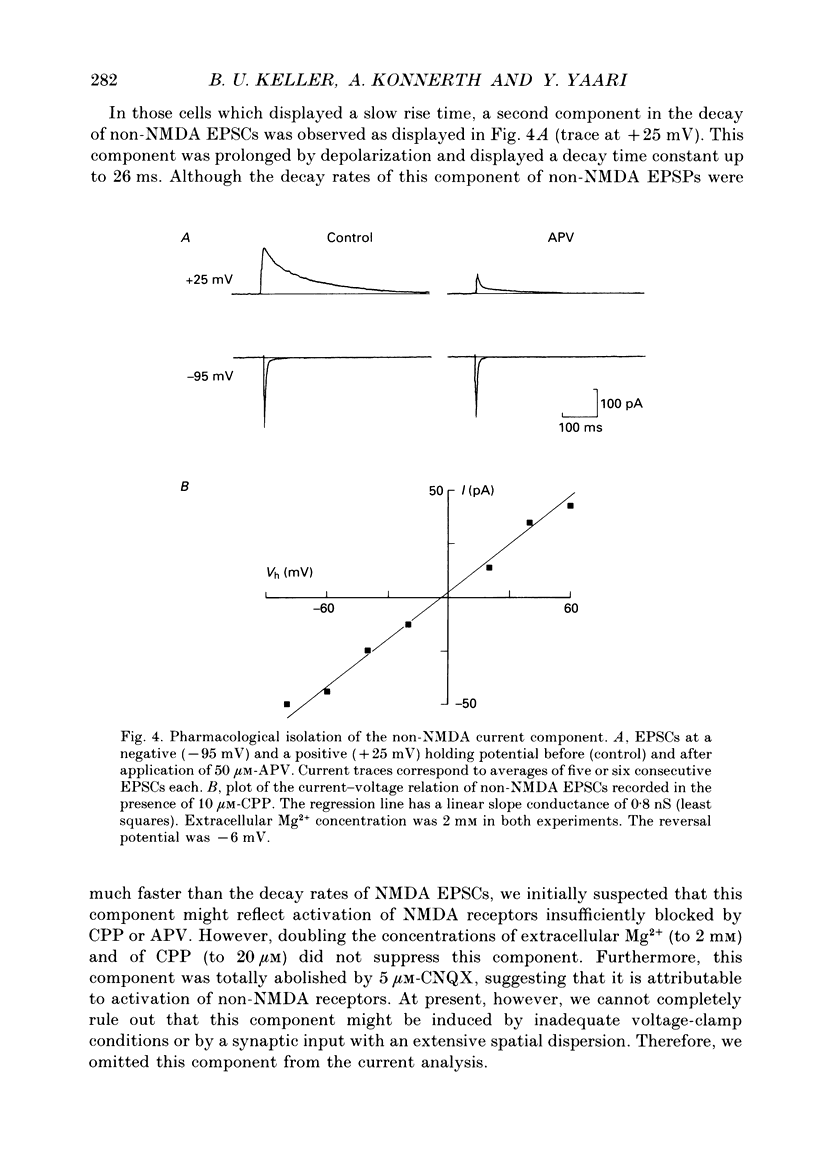







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreasen M., Lambert J. D., Jensen M. S. Direct demonstration of an N-methyl-D-aspartate receptor mediated component of excitatory synaptic transmission in area CA1 of the rat hippocampus. Neurosci Lett. 1988 Oct 31;93(1):61–66. doi: 10.1016/0304-3940(88)90013-4. [DOI] [PubMed] [Google Scholar]
- Andreasen M., Lambert J. D., Jensen M. S. Effects of new non-N-methyl-D-aspartate antagonists on synaptic transmission in the in vitro rat hippocampus. J Physiol. 1989 Jul;414:317–336. doi: 10.1113/jphysiol.1989.sp017690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Bregestovski P., Nowak L. N-methyl-D-aspartate-activated channels of mouse central neurones in magnesium-free solutions. J Physiol. 1988 May;399:207–226. doi: 10.1113/jphysiol.1988.sp017076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988 May;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers J. M., Stevens C. F. NMDA and non-NMDA receptors are co-localized at individual excitatory synapses in cultured rat hippocampus. Nature. 1989 Sep 21;341(6239):230–233. doi: 10.1038/341230a0. [DOI] [PubMed] [Google Scholar]
- Blake J. F., Brown M. W., Collingridge G. L. CNQX blocks acidic amino acid induced depolarizations and synaptic components mediated by non-NMDA receptors in rat hippocampal slices. Neurosci Lett. 1988 Jun 29;89(2):182–186. doi: 10.1016/0304-3940(88)90378-3. [DOI] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. W. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988 Oct;1(8):623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Herron C. E., Lester R. A. Frequency-dependent N-methyl-D-aspartate receptor-mediated synaptic transmission in rat hippocampus. J Physiol. 1988 May;399:301–312. doi: 10.1113/jphysiol.1988.sp017081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Herron C. E., Lester R. A. Synaptic activation of N-methyl-D-aspartate receptors in the Schaffer collateral-commissural pathway of rat hippocampus. J Physiol. 1988 May;399:283–300. doi: 10.1113/jphysiol.1988.sp017080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V., Forda S., Kelly J. S. The reversal potential of excitatory amino acid action on granule cells of the rat dentate gyrus. J Physiol. 1984 Jun;351:327–342. doi: 10.1113/jphysiol.1984.sp015248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. Multiple-conductance channels activated by excitatory amino acids in cerebellar neurons. Nature. 1987 Feb 5;325(6104):525–528. doi: 10.1038/325525a0. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. On the multiple-conductance single channels activated by excitatory amino acids in large cerebellar neurones of the rat. J Physiol. 1989 Aug;415:555–582. doi: 10.1113/jphysiol.1989.sp017736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. Whole-cell current noise produced by excitatory and inhibitory amino acids in large cerebellar neurones of the rat. J Physiol. 1989 Aug;415:533–553. doi: 10.1113/jphysiol.1989.sp017735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N., Grillner S. Dual-component synaptic potentials in the lamprey mediated by excitatory amino acid receptors. J Neurosci. 1986 Sep;6(9):2653–2661. doi: 10.1523/JNEUROSCI.06-09-02653.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudel J., Franke C., Hatt H., Ramsey R. L., Usherwood P. N. Rapid activation and desensitization by glutamate of excitatory, cation-selective channels in locust muscle. Neurosci Lett. 1988 May 16;88(1):33–38. doi: 10.1016/0304-3940(88)90311-4. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch-clamp study. J Physiol. 1990 Nov;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B., Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989 Sep;414(5):600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Forsythe I. D., Westbrook G. L. Slow excitatory postsynaptic currents mediated by N-methyl-D-aspartate receptors on cultured mouse central neurones. J Physiol. 1988 Feb;396:515–533. doi: 10.1113/jphysiol.1988.sp016975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke C., Hatt H., Dudel J. Liquid filament switch for ultra-fast exchanges of solutions at excised patches of synaptic membrane of crayfish muscle. Neurosci Lett. 1987 Jun 15;77(2):199–204. doi: 10.1016/0304-3940(87)90586-6. [DOI] [PubMed] [Google Scholar]
- Hablitz J. J., Langmoen I. A. Excitation of hippocampal pyramidal cells by glutamate in the guinea-pig and rat. J Physiol. 1982 Apr;325:317–331. doi: 10.1113/jphysiol.1982.sp014152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hestrin S., Nicoll R. A., Perkel D. J., Sah P. Analysis of excitatory synaptic action in pyramidal cells using whole-cell recording from rat hippocampal slices. J Physiol. 1990 Mar;422:203–225. doi: 10.1113/jphysiol.1990.sp017980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M., O'Shea-Greenfield A., Rogers S. W., Heinemann S. Cloning by functional expression of a member of the glutamate receptor family. Nature. 1989 Dec 7;342(6250):643–648. doi: 10.1038/342643a0. [DOI] [PubMed] [Google Scholar]
- Honoré T., Davies S. N., Drejer J., Fletcher E. J., Jacobsen P., Lodge D., Nielsen F. E. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988 Aug 5;241(4866):701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- Howe J. R., Colquhoun D., Cull-Candy S. G. On the kinetics of large-conductance glutamate-receptor ion channels in rat cerebellar granule neurons. Proc R Soc Lond B Biol Sci. 1988 May 23;233(1273):407–422. doi: 10.1098/rspb.1988.0030. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature. 1987 Feb 5;325(6104):522–525. doi: 10.1038/325522a0. [DOI] [PubMed] [Google Scholar]
- Jones R. S. Complex synaptic responses of entorhinal cortical cells in the rat to subicular stimulation in vitro: demonstration of an NMDA receptor-mediated component. Neurosci Lett. 1987 Oct 16;81(1-2):209–214. doi: 10.1016/0304-3940(87)90000-0. [DOI] [PubMed] [Google Scholar]
- Konnerth A., Keller B. U., Ballanyi K., Yaari Y. Voltage sensitivity of NMDA-receptor mediated postsynaptic currents. Exp Brain Res. 1990;81(1):209–212. doi: 10.1007/BF00230117. [DOI] [PubMed] [Google Scholar]
- Konnerth A., Keller B. U., Lev-Tov A. Patch clamp analysis of excitatory synapses in mammalian spinal cord slices. Pflugers Arch. 1990 Nov;417(3):285–290. doi: 10.1007/BF00370994. [DOI] [PubMed] [Google Scholar]
- Konnerth A., Llano I., Armstrong C. M. Synaptic currents in cerebellar Purkinje cells. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2662–2665. doi: 10.1073/pnas.87.7.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J. D., Jones R. S. Activation of N-methyl-D-aspartate receptors contributes to the EPSP at perforant path synapses in the rat dentate gyrus in vitro. Neurosci Lett. 1989 Feb 27;97(3):323–328. doi: 10.1016/0304-3940(89)90618-6. [DOI] [PubMed] [Google Scholar]
- Lester R. A., Clements J. D., Westbrook G. L., Jahr C. E. Channel kinetics determine the time course of NMDA receptor-mediated synaptic currents. Nature. 1990 Aug 9;346(6284):565–567. doi: 10.1038/346565a0. [DOI] [PubMed] [Google Scholar]
- Llano I., Marty A., Armstrong C. M., Konnerth A. Synaptic- and agonist-induced excitatory currents of Purkinje cells in rat cerebellar slices. J Physiol. 1991 Mar;434:183–213. doi: 10.1113/jphysiol.1991.sp018465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomo T. Patterns of activation in a monosynaptic cortical pathway: the perforant path input to the dentate area of the hippocampal formation. Exp Brain Res. 1971;12(1):18–45. [PubMed] [Google Scholar]
- MacDermott A. B., Mayer M. L., Westbrook G. L., Smith S. J., Barker J. L. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. 1986 May 29-Jun 4Nature. 321(6069):519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Mody I., Salter M. W., MacDonald J. F. Requirement of NMDA receptor/channels for intracellular high-energy phosphates and the extent of intraneuronal calcium buffering in cultured mouse hippocampal neurons. Neurosci Lett. 1988 Oct 31;93(1):73–78. doi: 10.1016/0304-3940(88)90015-8. [DOI] [PubMed] [Google Scholar]
- Mody I., Stanton P. K., Heinemann U. Activation of N-methyl-D-aspartate receptors parallels changes in cellular and synaptic properties of dentate gyrus granule cells after kindling. J Neurophysiol. 1988 Mar;59(3):1033–1054. doi: 10.1152/jn.1988.59.3.1033. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Bridges R. J., Cotman C. W. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Cotman C. W. Distribution of N-methyl-D-aspartate-sensitive L-[3H]glutamate-binding sites in rat brain. J Neurosci. 1985 Nov;5(11):2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson P. G., Pun R. Y., Westbrook G. L. Synaptic excitation in cultures of mouse spinal cord neurones: receptor pharmacology and behaviour of synaptic currents. J Physiol. 1986 Mar;372:169–190. doi: 10.1113/jphysiol.1986.sp016003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Salt T. E. Mediation of thalamic sensory input by both NMDA receptors and non-NMDA receptors. Nature. 1986 Jul 17;322(6076):263–265. doi: 10.1038/322263a0. [DOI] [PubMed] [Google Scholar]
- Tang C. M., Dichter M., Morad M. Quisqualate activates a rapidly inactivating high conductance ionic channel in hippocampal neurons. Science. 1989 Mar 17;243(4897):1474–1477. doi: 10.1126/science.2467378. [DOI] [PubMed] [Google Scholar]
- Thomson A. M. A magnesium-sensitive post-synaptic potential in rat cerebral cortex resembles neuronal responses to N-methylaspartate. J Physiol. 1986 Jan;370:531–549. doi: 10.1113/jphysiol.1986.sp015949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell L. O., Fischbach G. D. Glutamate receptor desensitization and its role in synaptic transmission. Neuron. 1989 Aug;3(2):209–218. doi: 10.1016/0896-6273(89)90034-2. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]



