Abstract
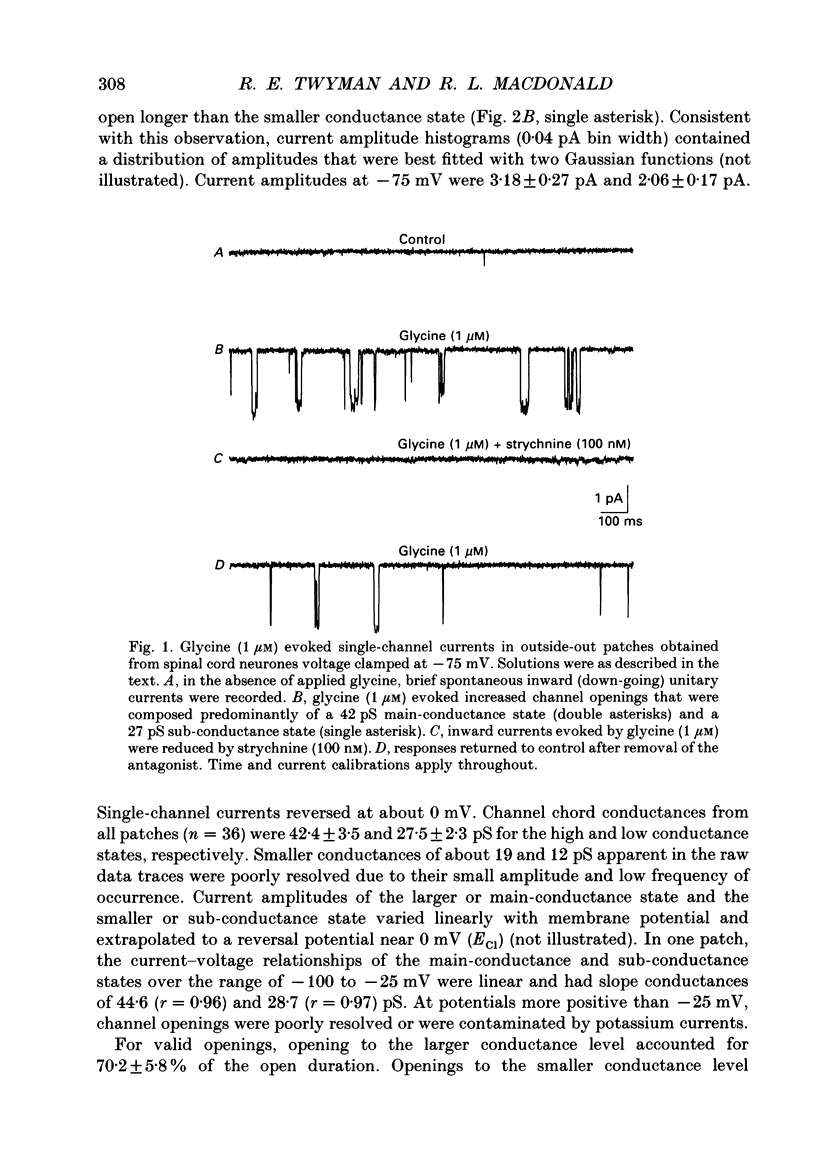
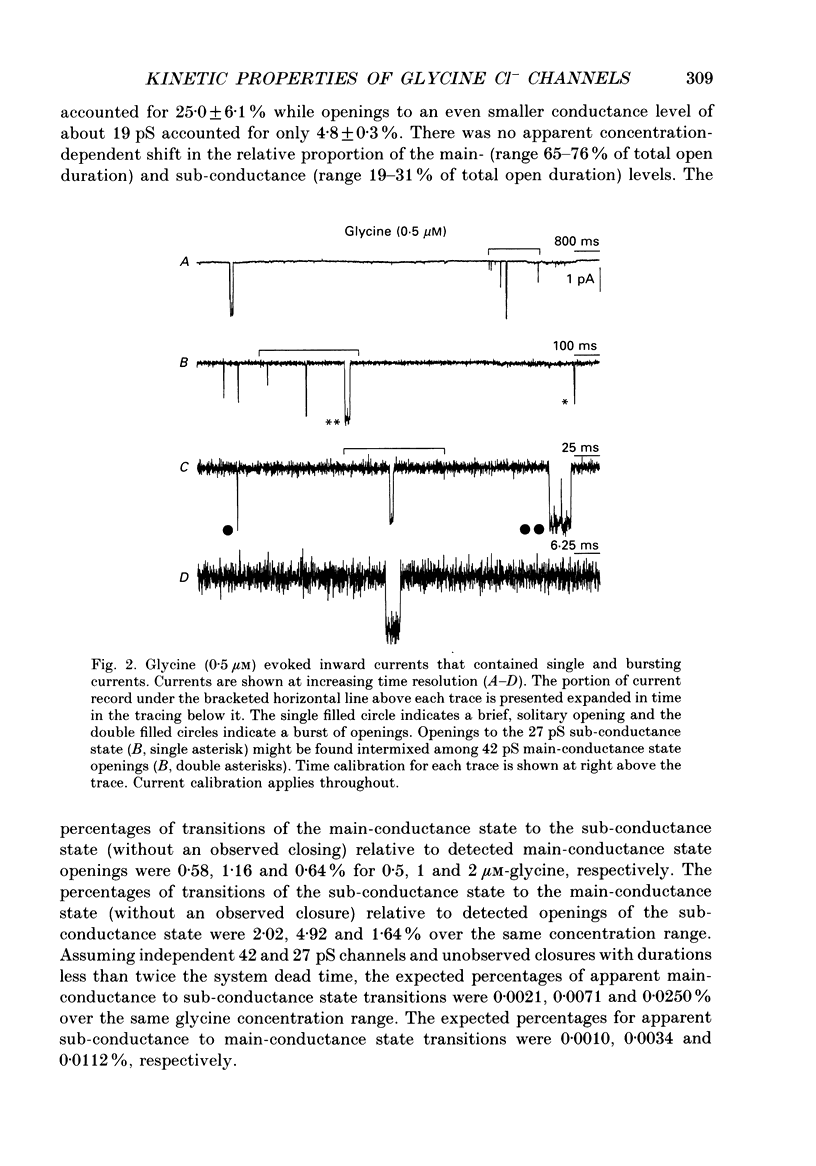
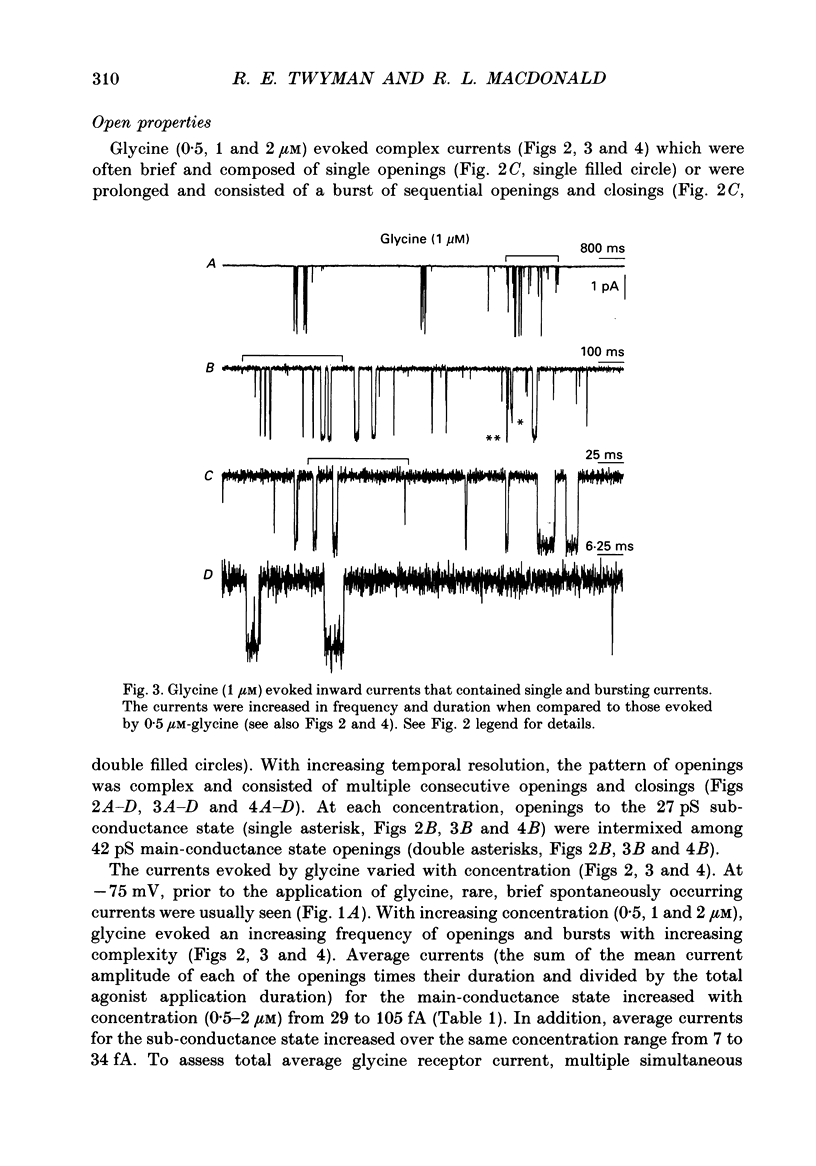
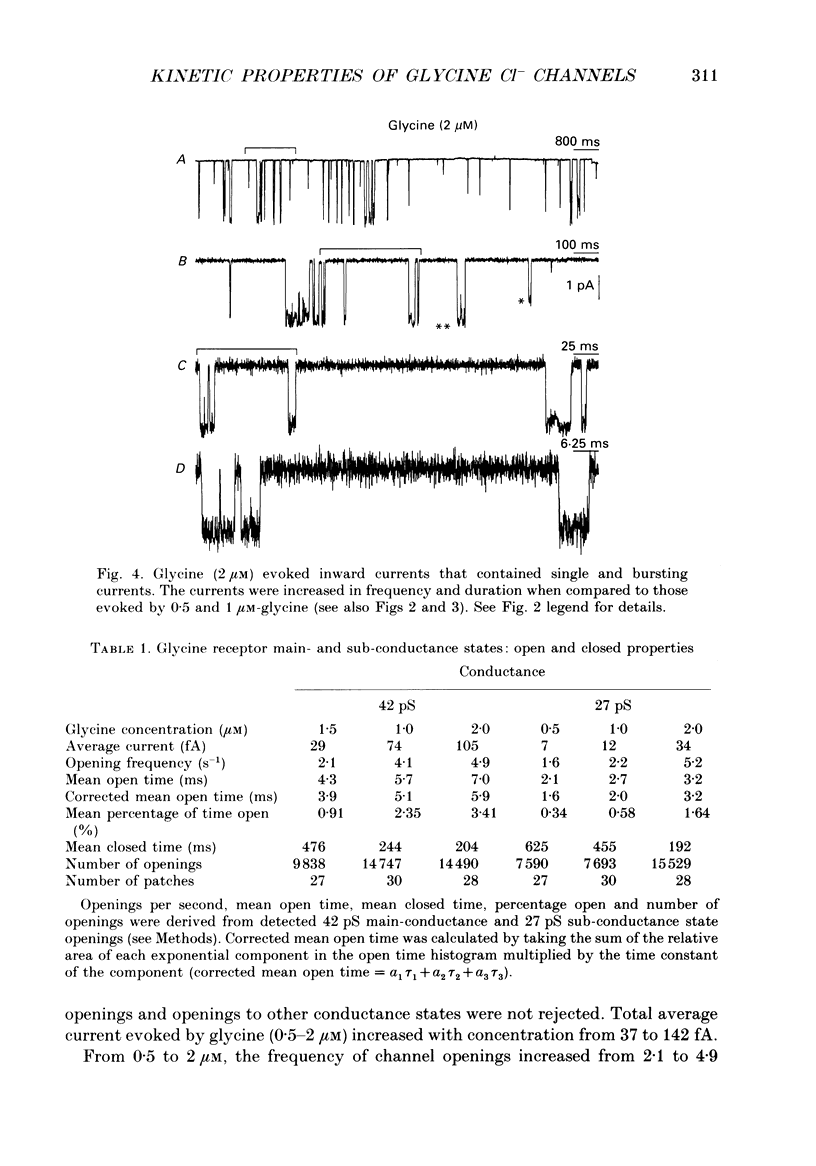
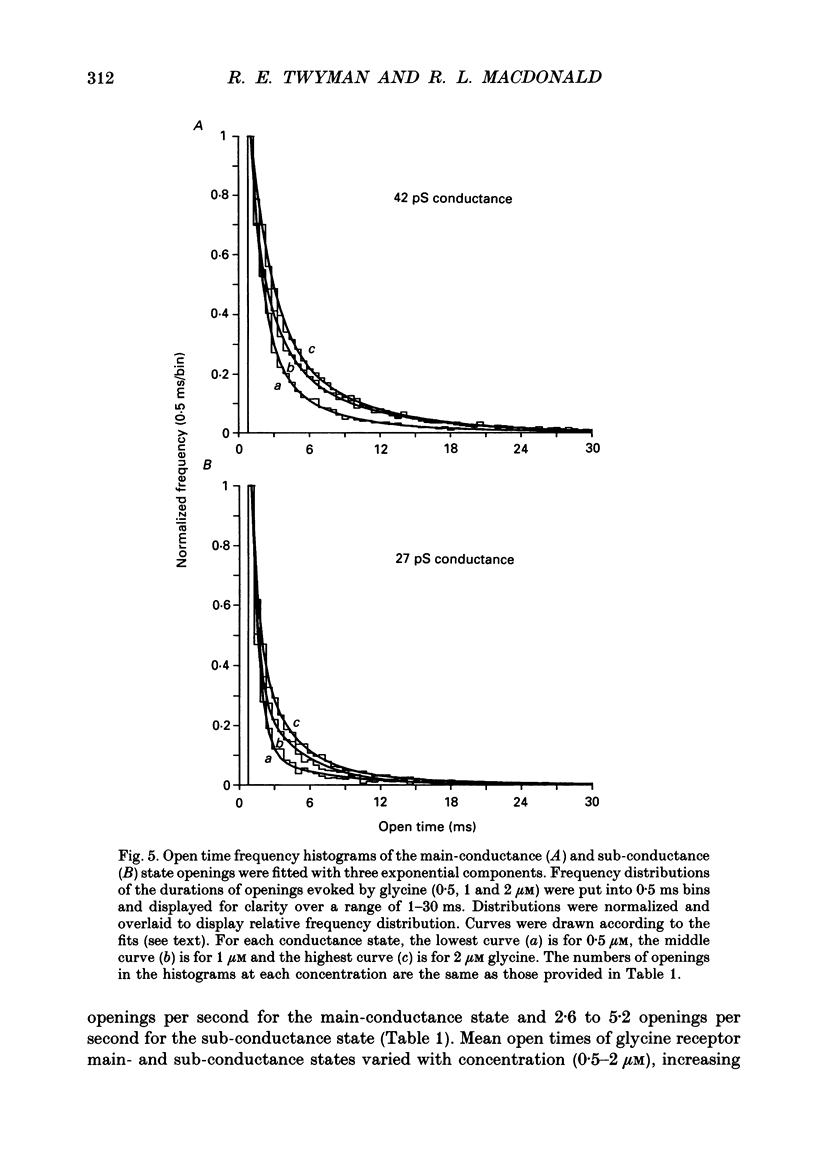
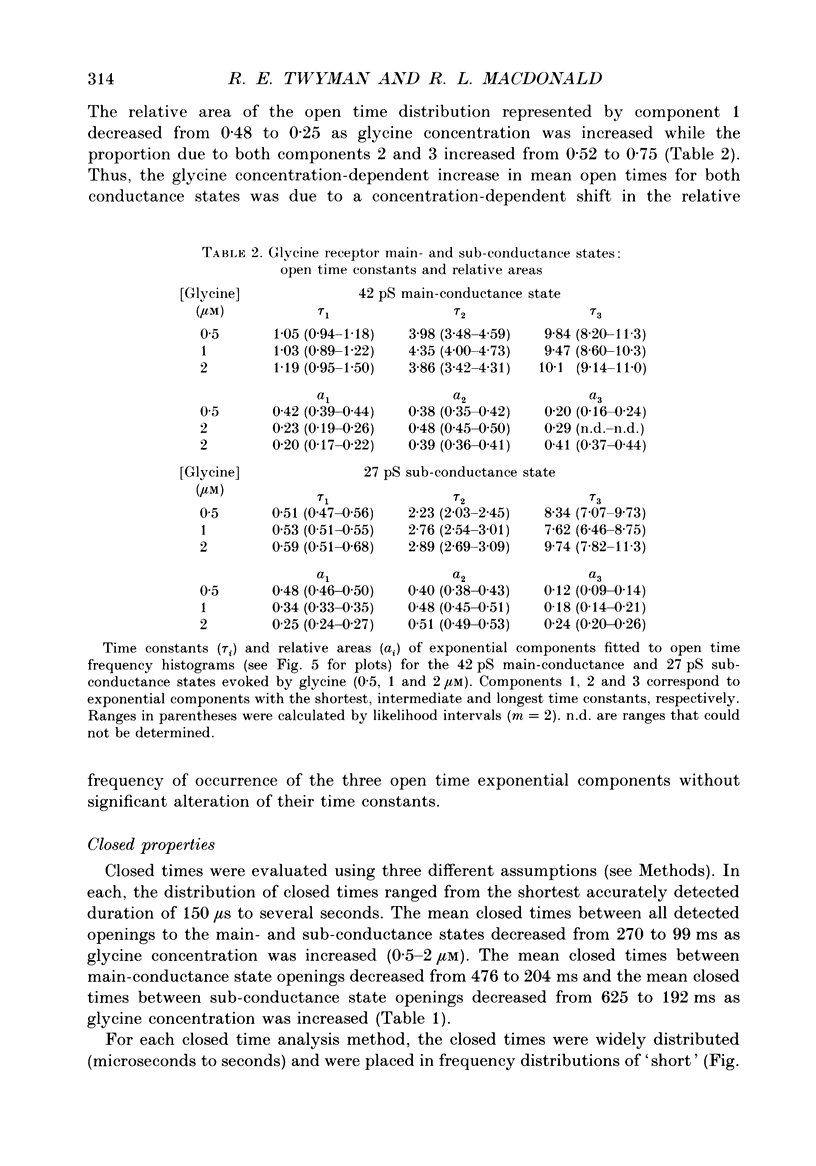
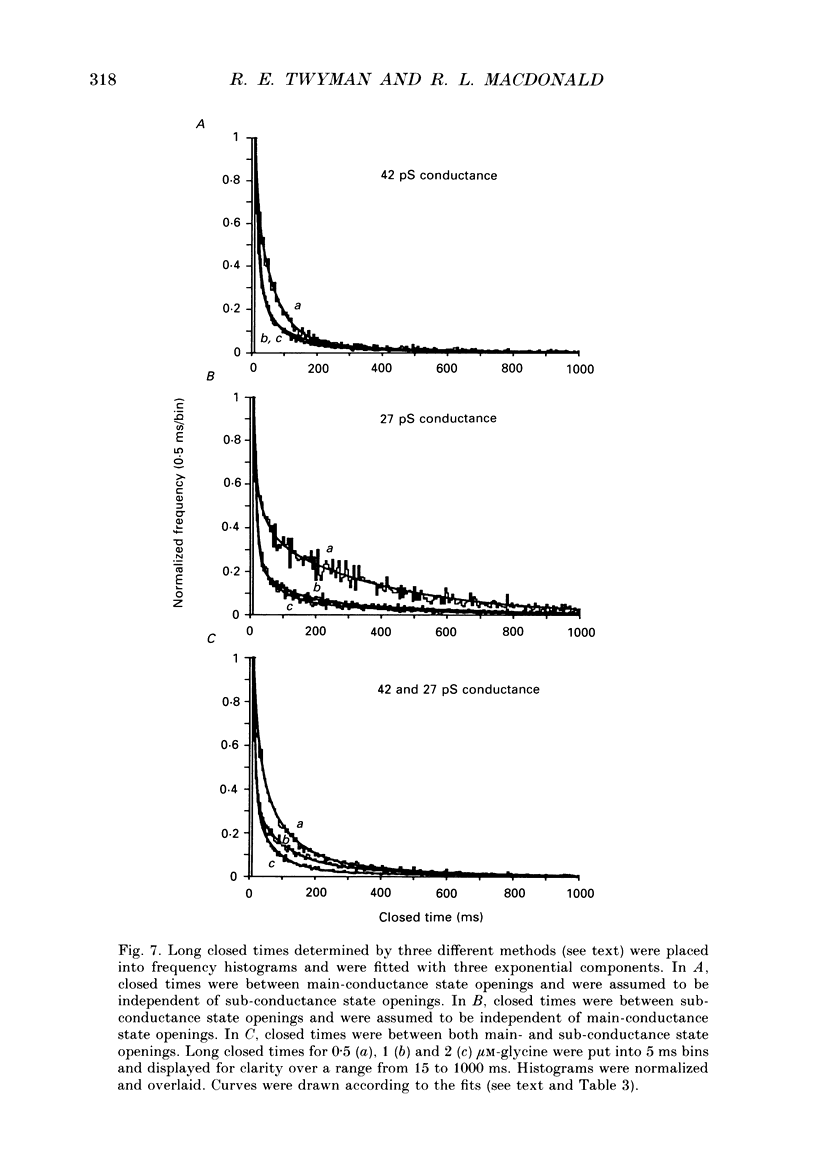
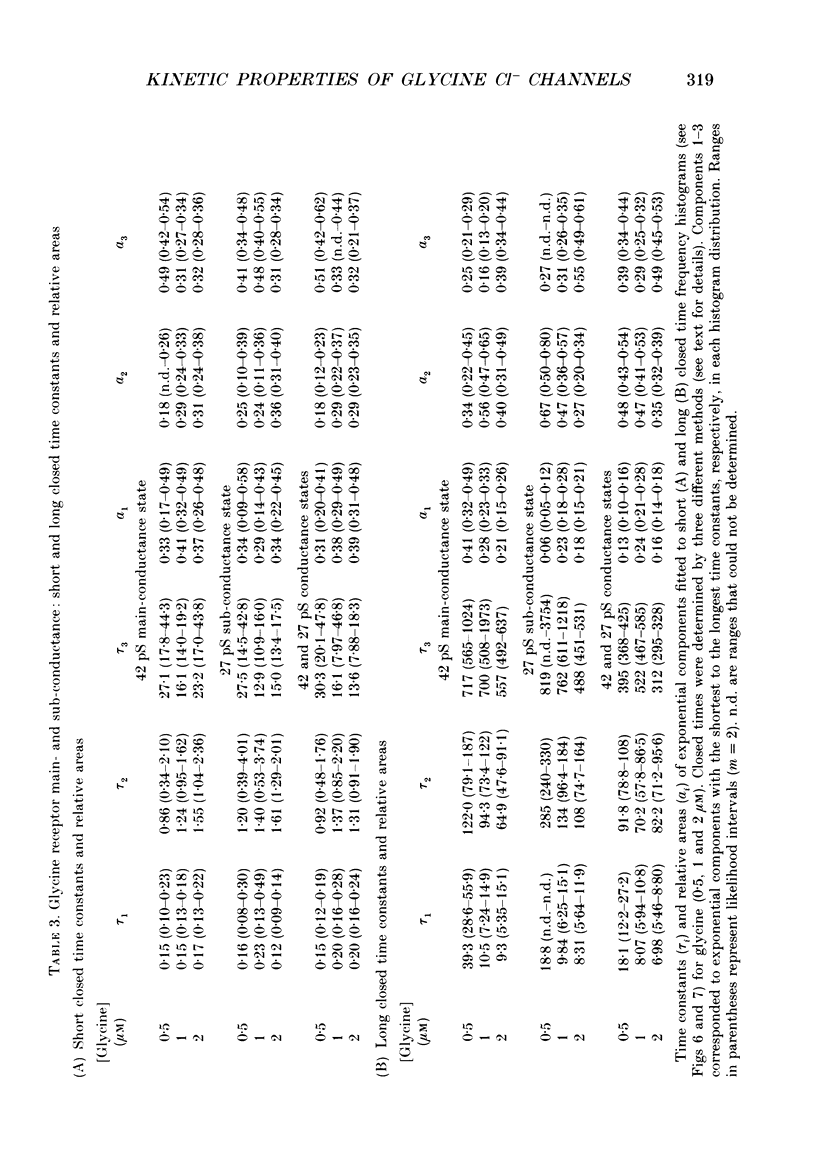
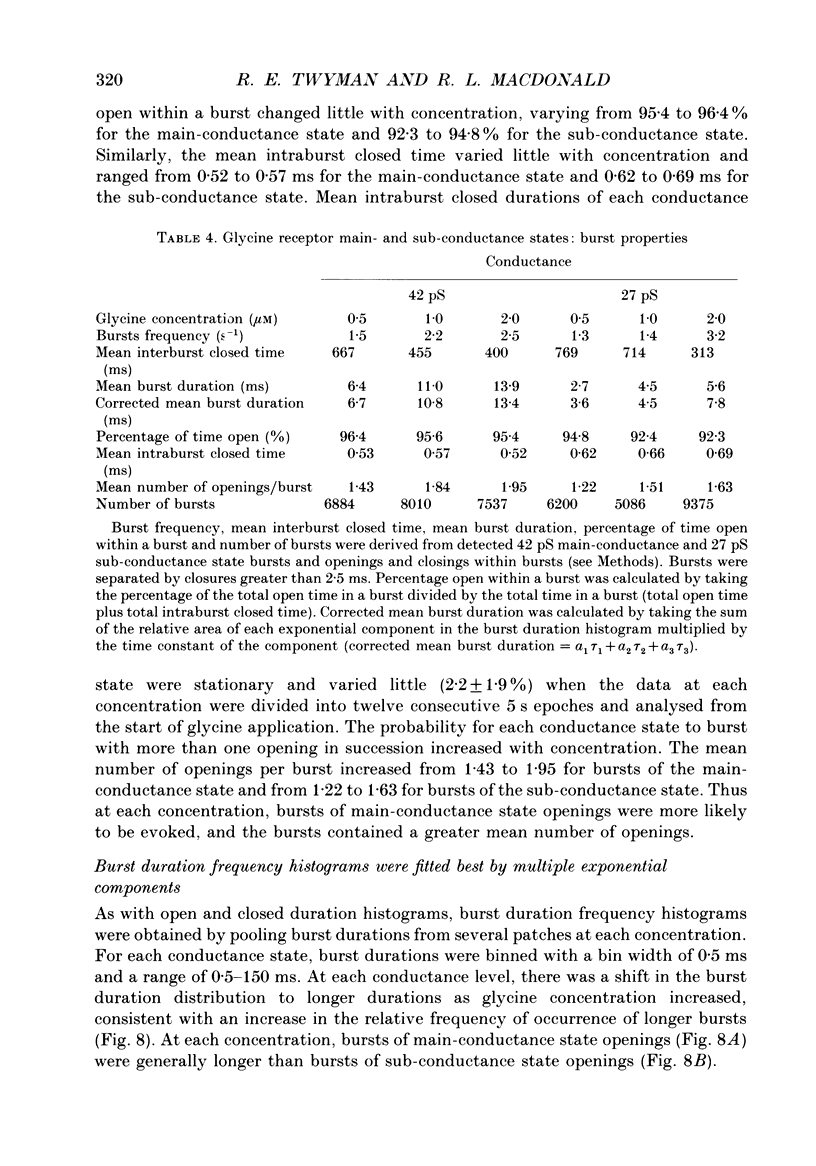
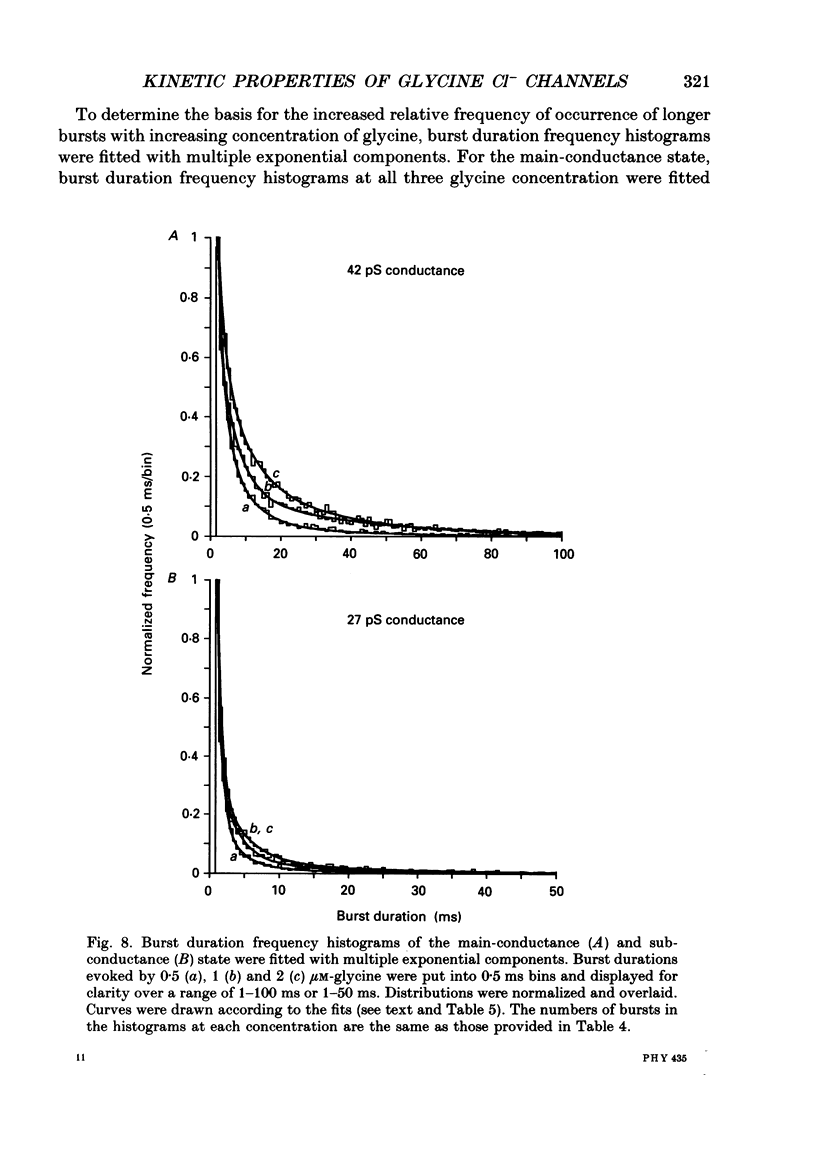
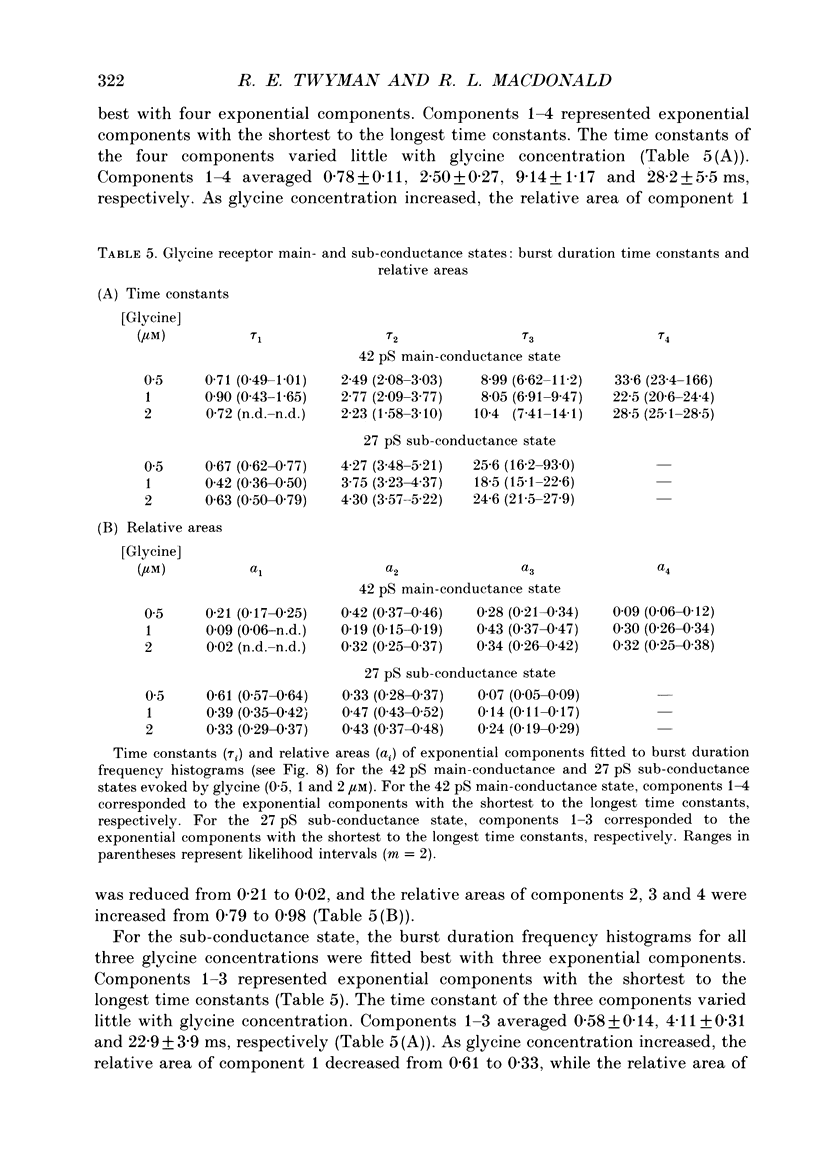
1. The kinetic properties of the two most frequent conductance states of glycine receptor channels from somata of mouse spinal cord neurones in cell culture were investigated using the outside-out patch clamp technique. At low concentrations of glycine (0.5, 1 and 2 microM), single-channel currents were recorded with two predominant amplitudes corresponding to a dominant or main-conductance state of about 42 pS and a sub-conductance state of about 27 pS. Both conductance states opened singly and in bursts of several openings. Total current evoked and single-channel opening frequency increased as glycine concentration was increased from 0.5 to 2 microM. 2. For both conductance states mean open times were increased and open time frequency histograms were shifted to longer times as glycine concentration was increased from 0.5 to 2 microM. For both conductance states, three exponential components were required to fit best open time frequency distribution histograms at all glycine concentrations (0.5, 1 and 2 microM). The time constants of the exponential components for each conductance state were not significantly different across concentration, suggesting that the main- and sub-conductance states of the channel each opened into at least three open states. For the main-conductance state, the time constants were 1.09 +/- 0.09, 4.06 +/- 0.26 and 9.79 +/- 0.30 ms. For the sub-conductance state, the time constants were 0.55 +/- 0.04, 2.64 +/- 0.35 and 8.57 +/- 1.08 ms. The increase in long open times with concentration was due primarily to a shift in relative frequency of occurrence of openings from the shortest to the two longest open states. 3. Closed time frequency distributions of closures between main-conductance state openings, closures between sub-conductance state openings and closures between both main- and sub-conductance state openings were fitted with multiple exponential components, suggesting that the channel had several closed states. The two shortest time constants (0.16 +/- 0.01 and 1.26 +/- 0.13 ms) did not vary significantly with concentration (0.5-2 microM) or method of analysis. The longer time constant varied with concentration. 4. Bursts were defined as groups of openings surrounded by closures greater than a critical closed time. For both conductances states, mean burst durations were increased and burst duration frequency histograms were shifted to longer times as glycine concentration was increased from 0.5 to 2 miroM. Burst duration frequency histograms contained four exponential components for the main-conductance state and three exponential components for the sub-conductance state.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF

















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Kaneda M. Glycine-gated chloride current in acutely isolated rat hypothalamic neurons. J Neurophysiol. 1989 Dec;62(6):1400–1409. doi: 10.1152/jn.1989.62.6.1400. [DOI] [PubMed] [Google Scholar]
- Aprison M. H., Werman R. The distribution of glycine in cat spinal cord and roots. Life Sci. 1965 Nov;4(21):2075–2083. doi: 10.1016/0024-3205(65)90325-5. [DOI] [PubMed] [Google Scholar]
- Barker J. L., McBurney R. N. GABA and glycine may share the same conductance channel on cultured mammalian neurones. Nature. 1979 Jan 18;277(5693):234–236. doi: 10.1038/277234a0. [DOI] [PubMed] [Google Scholar]
- Barker J. L., McBurney R. N., MacDonald J. F. Fluctuation analysis of neutral amino acid responses in cultured mouse spinal neurones. J Physiol. 1982 Jan;322:365–387. doi: 10.1113/jphysiol.1982.sp014042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., Ransom B. R. Amino acid pharmacology of mammalian central neurones grown in tissue culture. J Physiol. 1978 Jul;280:331–354. doi: 10.1113/jphysiol.1978.sp012387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C. M., Hermans-Borgmeyer I., Schmitt B., Betz H. The glycine receptor deficiency of the mutant mouse spastic: evidence for normal glycine receptor structure and localization. J Neurosci. 1986 May;6(5):1358–1364. doi: 10.1523/JNEUROSCI.06-05-01358.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Correcting single channel data for missed events. Biophys J. 1986 May;49(5):967–980. doi: 10.1016/S0006-3495(86)83725-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Miledi R., Parker I. Single glutamate-activated channels recorded from locust muscle fibres with perfused patch-clamp electrodes. J Physiol. 1981 Dec;321:195–210. doi: 10.1113/jphysiol.1981.sp013979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Hösli L., Johnston G. A. A pharmacological study of the depression of spinal neurones by glycine and related amino acids. Exp Brain Res. 1968;6(1):1–18. doi: 10.1007/BF00235443. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Hösli L., Johnston G. A., Johnston I. H. The hyperpolarization of spinal motoneurones by glycine and related amino acids. Exp Brain Res. 1968;5(3):235–258. doi: 10.1007/BF00238666. [DOI] [PubMed] [Google Scholar]
- Dionne V. E., Leibowitz M. D. Acetylcholine receptor kinetics. A description from single-channel currents at snake neuromuscular junctions. Biophys J. 1982 Sep;39(3):253–261. doi: 10.1016/S0006-3495(82)84515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne V. E., Steinbach J. H., Stevens C. F. An analysis of the dose-response relationship at voltage-clamped frog neuromuscular junctions. J Physiol. 1978 Aug;281:421–444. doi: 10.1113/jphysiol.1978.sp012431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M. R., Martin A. R. Analysis of glycine-activated inhibitory post-synaptic channels in brain-stem neurones of the lamprey. J Physiol. 1983 Sep;342:99–117. doi: 10.1113/jphysiol.1983.sp014842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenningloh G., Rienitz A., Schmitt B., Methfessel C., Zensen M., Beyreuther K., Gundelfinger E. D., Betz H. The strychnine-binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors. Nature. 1987 Jul 16;328(6127):215–220. doi: 10.1038/328215a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Bormann J., Sakmann B. Activation of multiple-conductance state chloride channels in spinal neurones by glycine and GABA. 1983 Oct 27-Nov 2Nature. 305(5937):805–808. doi: 10.1038/305805a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hestrin S., Korenbrot J. I., Maricq A. V. Kinetics of activation of acetylcholine receptors in a mouse muscle cell line under a range of acetylcholine concentrations. Biophys J. 1987 Mar;51(3):449–455. doi: 10.1016/S0006-3495(87)83366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B. Dependence of acetylcholine receptor channel kinetics on agonist concentration in cultured mouse muscle fibres. J Physiol. 1988 Mar;397:555–583. doi: 10.1113/jphysiol.1988.sp017019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo F., Schuetze S. M. Kinetic differences between embryonic- and adult-type acetylcholine receptors in rat myotubes. J Physiol. 1988 Feb;396:267–296. doi: 10.1113/jphysiol.1988.sp016962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca P., Montal M. S., Lindstrom J. M., Montal M. The occurrence of long openings in the purified cholinergic receptor channel increases with acetylcholine concentration. J Neurosci. 1985 Dec;5(12):3409–3413. doi: 10.1523/JNEUROSCI.05-12-03409.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langosch D., Thomas L., Betz H. Conserved quaternary structure of ligand-gated ion channels: the postsynaptic glycine receptor is a pentamer. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7394–7398. doi: 10.1073/pnas.85.19.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald R. L., Rogers C. J., Twyman R. E. Kinetic properties of the GABAA receptor main conductance state of mouse spinal cord neurones in culture. J Physiol. 1989 Mar;410:479–499. doi: 10.1113/jphysiol.1989.sp017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers D. A., Barker J. L. GABA- and glycine-induced Cl- channels in cultured mouse spinal neurons require the same energy to close. Brain Res. 1981 Nov 16;224(2):441–445. doi: 10.1016/0006-8993(81)90875-1. [DOI] [PubMed] [Google Scholar]
- Mathers D. A. Spontaneous and GABA-induced single channel currents in cultured murine spinal cord neurons. Can J Physiol Pharmacol. 1985 Oct;63(10):1228–1233. doi: 10.1139/y85-203. [DOI] [PubMed] [Google Scholar]
- McManus O. B., Blatz A. L., Magleby K. L. Sampling, log binning, fitting, and plotting durations of open and shut intervals from single channels and the effects of noise. Pflugers Arch. 1987 Nov;410(4-5):530–553. doi: 10.1007/BF00586537. [DOI] [PubMed] [Google Scholar]
- Mishina M., Takai T., Imoto K., Noda M., Takahashi T., Numa S., Methfessel C., Sakmann B. Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature. 1986 May 22;321(6068):406–411. doi: 10.1038/321406a0. [DOI] [PubMed] [Google Scholar]
- Moss S. J., Smart T. G., Porter N. M., Nayeem N., Devine J., Stephenson F. A., Macdonald R. L., Barnard E. A. Cloned GABA receptors are maintained in a stable cell line: allosteric and channel properties. Eur J Pharmacol. 1990 Jul 31;189(1):77–88. doi: 10.1016/0922-4106(90)90232-m. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Padjen A., Barker J. L. Analysis of amino acid responses on frog motoneurones. Neuropharmacology. 1976 Jan;15(1):45–53. doi: 10.1016/0028-3908(76)90096-4. [DOI] [PubMed] [Google Scholar]
- Nowak L. M., Young A. B., Macdonald R. L. GABA and bicuculline actions on mouse spinal cord and cortical neurons in cell culture. Brain Res. 1982 Jul 22;244(1):155–164. doi: 10.1016/0006-8993(82)90913-1. [DOI] [PubMed] [Google Scholar]
- Pfeiffer F., Graham D., Betz H. Purification by affinity chromatography of the glycine receptor of rat spinal cord. J Biol Chem. 1982 Aug 25;257(16):9389–9393. [PubMed] [Google Scholar]
- Pritchett D. B., Sontheimer H., Shivers B. D., Ymer S., Kettenmann H., Schofield P. R., Seeburg P. H. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989 Apr 13;338(6216):582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Hamill O. P., Bormann J. Patch-clamp measurements of elementary chloride currents activated by the putative inhibitory transmitter GABA and glycine in mammalian spinal neurons. J Neural Transm Suppl. 1983;18:83–95. [PubMed] [Google Scholar]
- Sakmann B., Methfessel C., Mishina M., Takahashi T., Takai T., Kurasaki M., Fukuda K., Numa S. Role of acetylcholine receptor subunits in gating of the channel. Nature. 1985 Dec 12;318(6046):538–543. doi: 10.1038/318538a0. [DOI] [PubMed] [Google Scholar]
- Schmieden V., Grenningloh G., Schofield P. R., Betz H. Functional expression in Xenopus oocytes of the strychnine binding 48 kd subunit of the glycine receptor. EMBO J. 1989 Mar;8(3):695–700. doi: 10.1002/j.1460-2075.1989.tb03428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield P. R., Darlison M. G., Fujita N., Burt D. R., Stephenson F. A., Rodriguez H., Rhee L. M., Ramachandran J., Reale V., Glencorse T. A. Sequence and functional expression of the GABA A receptor shows a ligand-gated receptor super-family. Nature. 1987 Jul 16;328(6127):221–227. doi: 10.1038/328221a0. [DOI] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Activation of a nicotinic acetylcholine receptor. Biophys J. 1984 Jan;45(1):175–185. doi: 10.1016/S0006-3495(84)84146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Activation of acetylcholine receptors on clonal mammalian BC3H-1 cells by low concentrations of agonist. J Physiol. 1986 Apr;373:129–162. doi: 10.1113/jphysiol.1986.sp016039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M., Zorec R., McBurney R. N. Conductance states activated by glycine and GABA in rat cultured spinal neurones. J Membr Biol. 1989 Apr;108(1):45–52. doi: 10.1007/BF01870424. [DOI] [PubMed] [Google Scholar]
- Takeda K., Trautmann A. A patch-clamp study of the partial agonist actions of tubocurarine on rat myotubes. J Physiol. 1984 Apr;349:353–374. doi: 10.1113/jphysiol.1984.sp015160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolliver J. M., Pellmar T. C. Effects of dithiothreitol, a sulfhydryl reducing agent, on CA1 pyramidal cells of the guinea pig hippocampus in vitro. Brain Res. 1988 Jul 19;456(1):49–56. doi: 10.1016/0006-8993(88)90345-9. [DOI] [PubMed] [Google Scholar]
- Twyman R. E., Rogers C. J., Macdonald R. L. Intraburst kinetic properties of the GABAA receptor main conductance state of mouse spinal cord neurones in culture. J Physiol. 1990 Apr;423:193–220. doi: 10.1113/jphysiol.1990.sp018018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werman R., Davidoff R. A., Aprison M. H. Inhibition of motoneurones by iontophoresis of glycine. Nature. 1967 May 13;214(5089):681–683. doi: 10.1038/214681a0. [DOI] [PubMed] [Google Scholar]
- Young A. B., Snyder S. H. The glycine synaptic receptor: evidence that strychnine binding is associated with the ionic conductance mechanism. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4002–4005. doi: 10.1073/pnas.71.10.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]


