Abstract
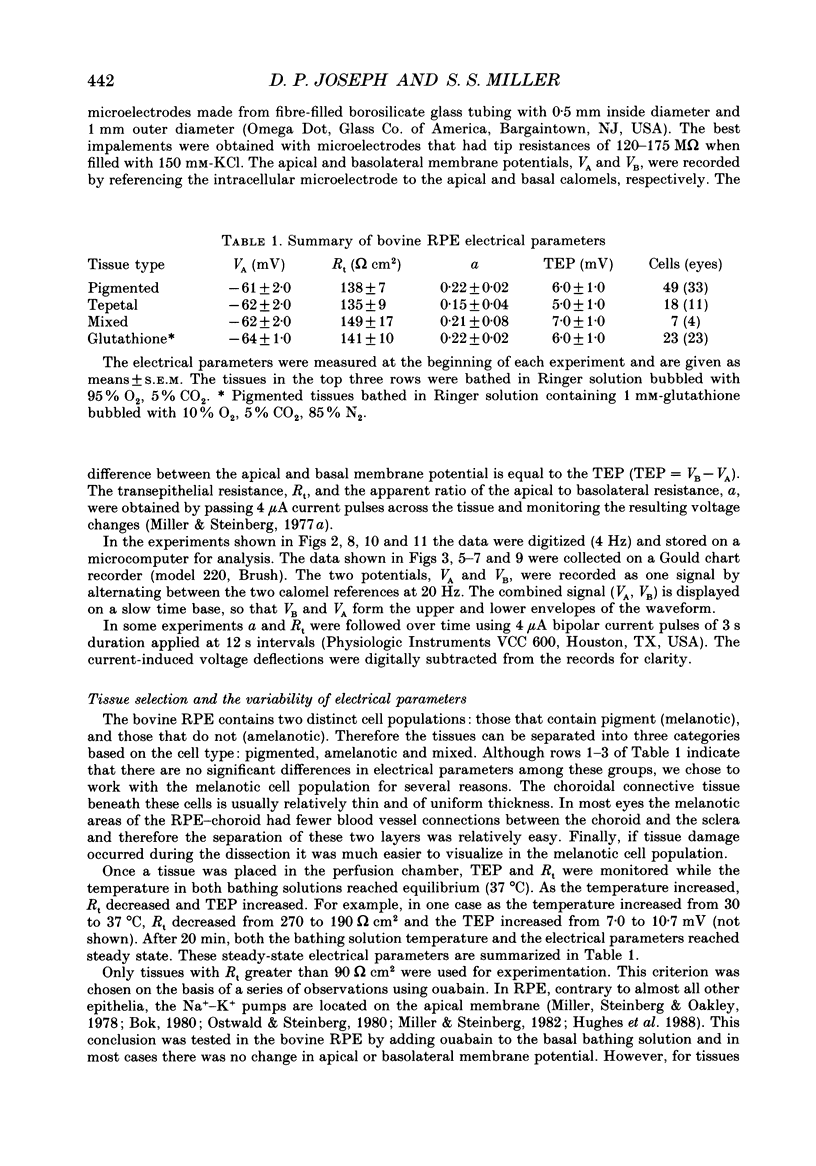
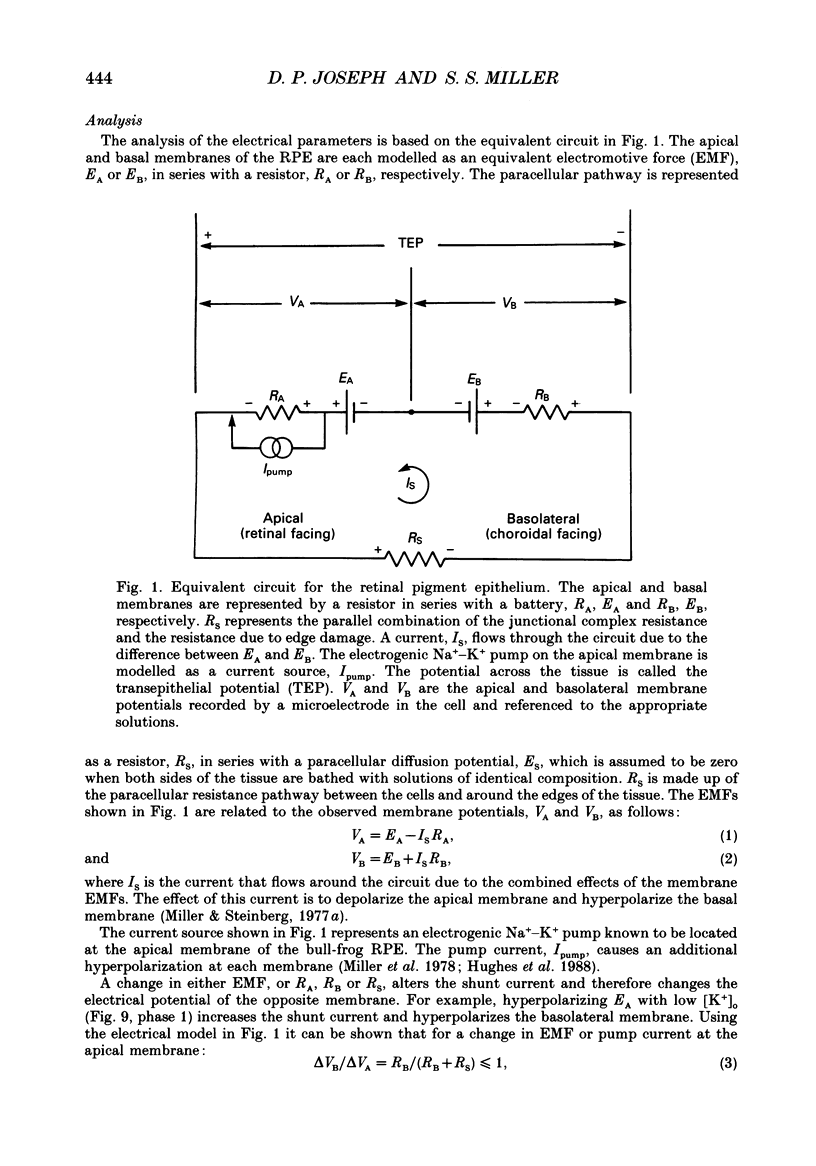
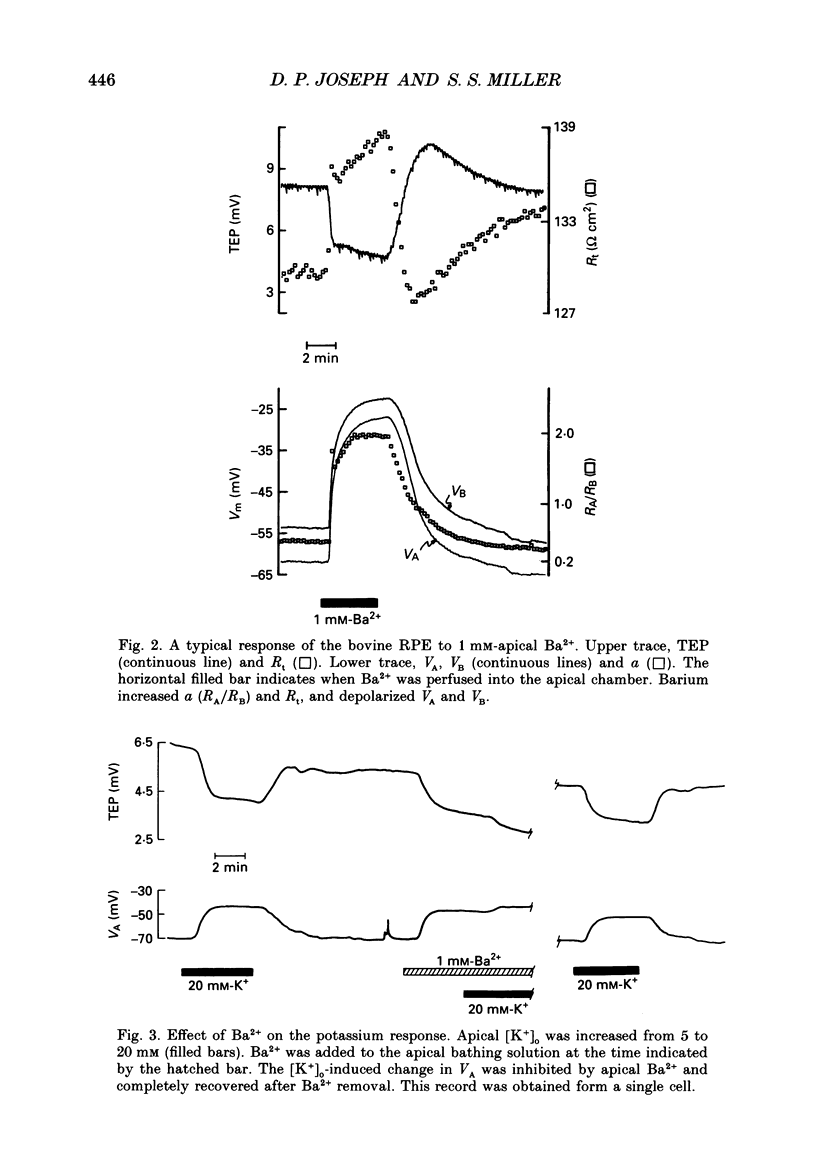
1. Intracellular voltage recordings using conventional and double-barrelled chloride-selective microelectrodes have been used to identify several transport mechanisms at the apical and basolateral membranes of the isolated bovine retinal pigment epithelium (RPE)-choroid preparation. Intracellular recordings were obtained from two cell populations, melanotic (pigmented) and amelanotic (non-pigmented). The electrical properties of these two populations are practically identical. For melanotic cells the average apical resting membrane potential (VA) is -61 +/- 2 mV (mean +/- S.E.M., n = 49 cells, thirty-three eyes). For these cells the ratio of apical to basolateral membrane resistance (a) was 0.22 +/- 0.02. The mean transepithelial voltage and resistance were 6 +/- 1 mV and 138 +/- 7 omega cm2, respectively. 2. The apical membrane, which faces the distal retina, contains a Ba(2+)-inhibitable K+ conductance and a ouabain-inhibitable, electrogenic Na(+)-K+ pump. In addition it contains a bumetanide-sensitive mechanism, the putative Na(+)-K(+)-Cl- cotransporter. The basolateral membrane contains a DIDS (4,4'-diisothiocyanostilbene-2,2'-disulphonic acid)-inhibitable chloride channel. The relative conductances of the apical and basolateral membranes to K+ and Cl- are TK approximately 0.9 and TCl approximately 0.7, respectively. 3. The ouabain-induced fast phase of apical membrane depolarization (0-30 s) was used to calculate the equivalent resistances of the apical (RA) and basolateral (RB) cell membranes, as well as the paracellular or shunt resistance (RS). They are: 3190 +/- 400, 17920 +/- 2730 and 2550 +/- 200 omega (mean +/- S.E.M., n = 9 tissues), respectively. From these data the equivalent electromotive forces (EMF) at the apical (EA) and basolateral (EB) membranes were also calculated. They are: -69 +/- 5.0 and -24 +/- 5.0 mV, respectively. 4. Intracellular Cl- activity (aiCl) was measured using double-barreled ion-selective microelectrodes. In the steady state aiCl = 61 +/- 4.0 mM and the Nernst potential ECl = -13.5 +/- 1.5 mV (mean +/- S.E.M., n = 4). 5. In the intact eye or in retina, RPE-choroid preparations it has been shown that the transition between light and dark alters the K+ concentration in the extracellular (or subretinal) space between the photoreceptors and the apical membrane of the RPE. These light-induced changes in subretinal [K+]o were qualitatively simulated in vitro by altering apical K+ between 5 and 2 mM. This produced a sequence of voltage changes at the apical and basolateral membranes that had three operationally distinct phases. Phase 1 is generated by the combination of an apical membrane K+ diffusion potential and inhibition of the electrogenic Na(+)-K+ pump.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





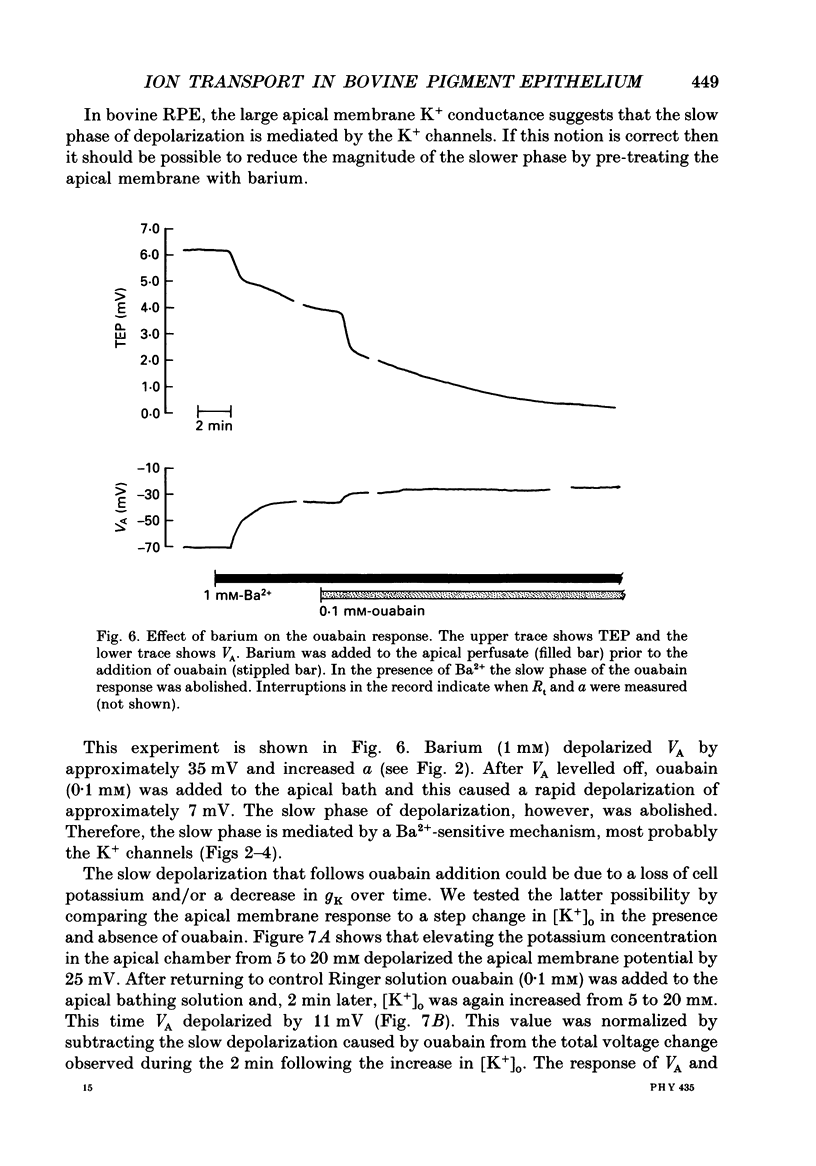




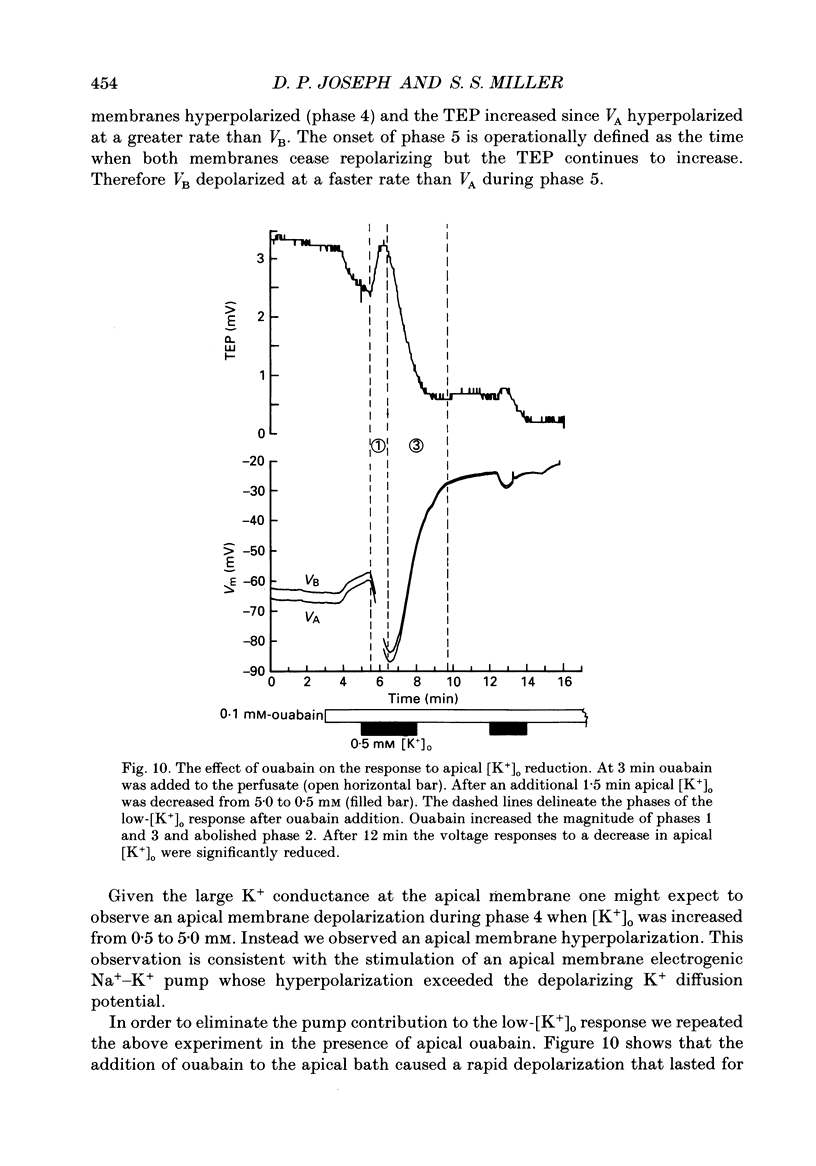
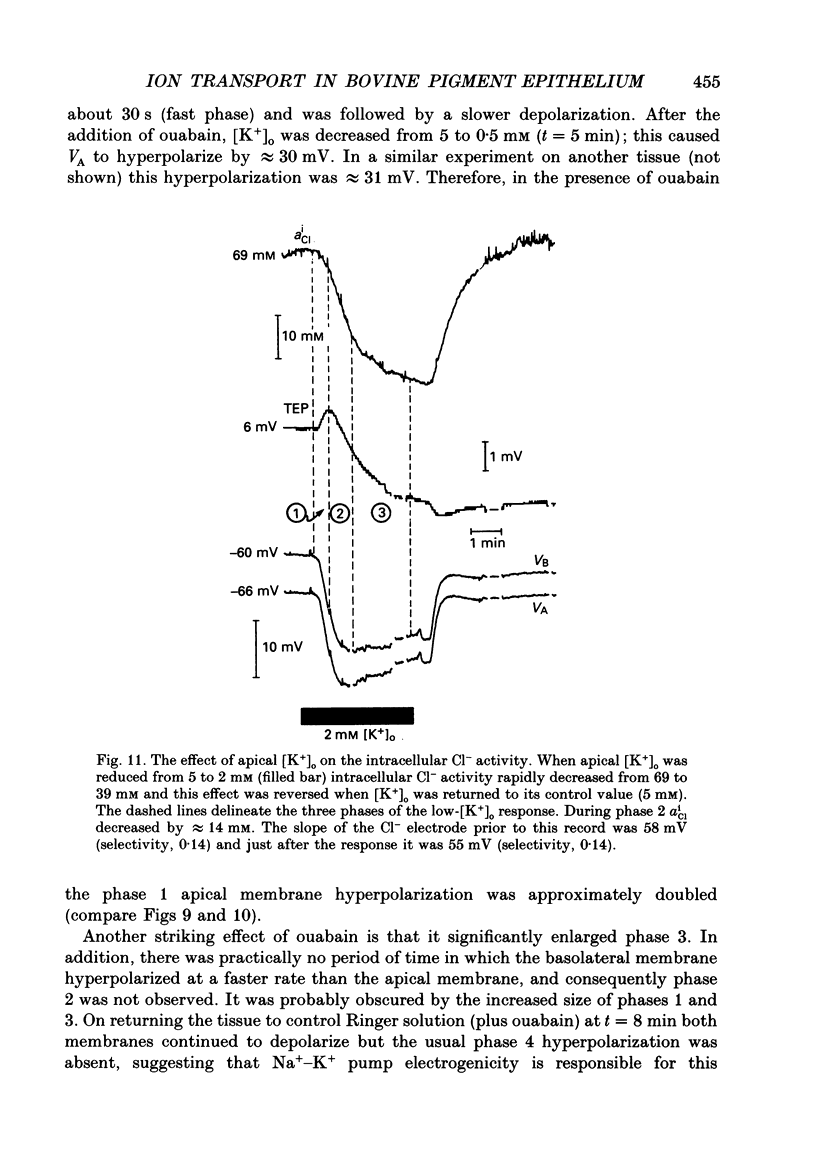








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adorante J. S., Miller S. S. Potassium-dependent volume regulation in retinal pigment epithelium is mediated by Na,K,Cl cotransport. J Gen Physiol. 1990 Dec;96(6):1153–1176. doi: 10.1085/jgp.96.6.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles J. A., Tsacopoulos M. A method of making fine double-barrelled potassium-sensitive micro-electrodes for intracellular recording [proceedings]. J Physiol. 1977 Aug;270(1):12P–14P. [PubMed] [Google Scholar]
- Davis C. W., Finn A. L. Sodium transport inhibition by amiloride reduces basolateral membrane potassium conductance in tight epithelia. Science. 1982 Apr 30;216(4545):525–527. doi: 10.1126/science.7071599. [DOI] [PubMed] [Google Scholar]
- Dikstein S., Maurice D. M. The metabolic basis to the fluid pump in the cornea. J Physiol. 1972 Feb;221(1):29–41. doi: 10.1113/jphysiol.1972.sp009736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton D. C., Brodwick M. S. Effects of barium on the potassium conductance of squid axon. J Gen Physiol. 1980 Jun;75(6):727–750. doi: 10.1085/jgp.75.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallemore R. P., Steinberg R. H. Effects of DIDS on the chick retinal pigment epithelium. I. Membrane potentials, apparent resistances, and mechanisms. J Neurosci. 1989 Jun;9(6):1968–1976. doi: 10.1523/JNEUROSCI.09-06-01968.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griff E. R., Shirao Y., Steinberg R. H. Ba2+ unmasks K+ modulation of the Na+-K+ pump in the frog retinal pigment epithelium. J Gen Physiol. 1985 Dec;86(6):853–876. doi: 10.1085/jgp.86.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griff E. R., Steinberg R. H. Changes in apical [K+] produce delayed basal membrane responses of the retinal pigment epithelium in the gecko. J Gen Physiol. 1984 Feb;83(2):193–211. doi: 10.1085/jgp.83.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halm D. R., Krasny E. J., Jr, Frizzell R. A. Electrophysiology of flounder intestinal mucosa. I. Conductance properties of the cellular and paracellular pathways. J Gen Physiol. 1985 Jun;85(6):843–864. doi: 10.1085/jgp.85.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanrahan J. W., Alles W. P., Lewis S. A. Single anion-selective channels in basolateral membrane of a mammalian tight epithelium. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7791–7795. doi: 10.1073/pnas.82.22.7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes B. A., Adorante J. S., Miller S. S., Lin H. Apical electrogenic NaHCO3 cotransport. A mechanism for HCO3 absorption across the retinal pigment epithelium. J Gen Physiol. 1989 Jul;94(1):125–150. doi: 10.1085/jgp.94.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes B. A., Miller S. S., Joseph D. P., Edelman J. L. cAMP stimulates the Na+-K+ pump in frog retinal pigment epithelium. Am J Physiol. 1988 Jan;254(1 Pt 1):C84–C98. doi: 10.1152/ajpcell.1988.254.1.C84. [DOI] [PubMed] [Google Scholar]
- Hughes B. A., Miller S. S., Machen T. E. Effects of cyclic AMP on fluid absorption and ion transport across frog retinal pigment epithelium. Measurements in the open-circuit state. J Gen Physiol. 1984 Jun;83(6):875–899. doi: 10.1085/jgp.83.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immel J., Steinberg R. H. Spatial buffering of K+ by the retinal pigment epithelium in frog. J Neurosci. 1986 Nov;6(11):3197–3204. doi: 10.1523/JNEUROSCI.06-11-03197.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue I. Voltage-dependent chloride conductance of the squid axon membrane and its blockade by some disulfonic stilbene derivatives. J Gen Physiol. 1985 Apr;85(4):519–537. doi: 10.1085/jgp.85.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy B. G. Na(+)-K(4)-Cl- cotransport in cultured cells derived from human retinal pigment epithelium. Am J Physiol. 1990 Jul;259(1 Pt 1):C29–C34. doi: 10.1152/ajpcell.1990.259.1.C29. [DOI] [PubMed] [Google Scholar]
- La Cour M. Rheogenic sodium-bicarbonate co-transport across the retinal membrane of the frog retinal pigment epithelium. J Physiol. 1989 Dec;419:539–553. doi: 10.1113/jphysiol.1989.sp017885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasansky A., De Fisch F. W. Potential, current, and ionic fluxes across the isolated retinal pigment epithelium and choriod. J Gen Physiol. 1966 May;49(5):913–924. doi: 10.1085/jgp.49.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenmeier R. A. Effects of light and darkness on oxygen distribution and consumption in the cat retina. J Gen Physiol. 1986 Oct;88(4):521–542. doi: 10.1085/jgp.88.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenmeier R. A., Steinberg R. H. Delayed basal hyperpolarization of cat retinal pigment epithelium and its relation to the fast oscillation of the DC electroretinogram. J Gen Physiol. 1984 Feb;83(2):213–232. doi: 10.1085/jgp.83.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenmeier R. A., Steinberg R. H. Mechanisms of hypoxic effects on the cat DC electroretinogram. Invest Ophthalmol Vis Sci. 1986 Sep;27(9):1385–1394. [PubMed] [Google Scholar]
- Matsumura Y., Cohen B., Guggino W. B., Giebisch G. Regulation of the basolateral potassium conductance of the Necturus proximal tubule. J Membr Biol. 1984;79(2):153–161. doi: 10.1007/BF01872119. [DOI] [PubMed] [Google Scholar]
- Messner G., Wang W., Paulmichl M., Oberleithner H., Lang F. Ouabain decreases apparent potassium-conductance in proximal tubules of the amphibian kidney. Pflugers Arch. 1985 May;404(2):131–137. doi: 10.1007/BF00585408. [DOI] [PubMed] [Google Scholar]
- Miller S. S., Edelman J. L. Active ion transport pathways in the bovine retinal pigment epithelium. J Physiol. 1990 May;424:283–300. doi: 10.1113/jphysiol.1990.sp018067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. S., Steinberg R. H. Active transport of ions across frog retinal pigment epithelium. Exp Eye Res. 1977 Sep;25(3):235–248. doi: 10.1016/0014-4835(77)90090-2. [DOI] [PubMed] [Google Scholar]
- Miller S. S., Steinberg R. H., Oakley B., 2nd The electrogenic sodium pump of the frog retinal pigment epithelium. J Membr Biol. 1978 Dec 29;44(3-4):259–279. doi: 10.1007/BF01944224. [DOI] [PubMed] [Google Scholar]
- Miller S. S., Steinberg R. H. Passive ionic properties of frog retinal pigment epithelium. J Membr Biol. 1977 Sep 15;36(4):337–372. doi: 10.1007/BF01868158. [DOI] [PubMed] [Google Scholar]
- Miller S. S., Steinberg R. H. Potassium modulation of taurine transport across the frog retinal pigment epithelium. J Gen Physiol. 1979 Aug;74(2):237–259. doi: 10.1085/jgp.74.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. S., Steinberg R. H. Potassium transport across the frog retinal pigment epithelium. J Membr Biol. 1982;67(3):199–209. doi: 10.1007/BF01868661. [DOI] [PubMed] [Google Scholar]
- Miller S., Farber D. Cyclic AMP modulation of ion transport across frog retinal pigment epithelium. Measurements in the short-circuit state. J Gen Physiol. 1984 Jun;83(6):853–874. doi: 10.1085/jgp.83.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montrose M., Randles J., Kimmich G. A. SITS-sensitive Cl- conductance pathway in chick intestinal cells. Am J Physiol. 1987 Nov;253(5 Pt 1):C693–C699. doi: 10.1152/ajpcell.1987.253.5.C693. [DOI] [PubMed] [Google Scholar]
- Newman E. A. Membrane physiology of retinal glial (Müller) cells. J Neurosci. 1985 Aug;5(8):2225–2239. doi: 10.1523/JNEUROSCI.05-08-02225.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Grady S. M., Palfrey H. C., Field M. Characteristics and functions of Na-K-Cl cotransport in epithelial tissues. Am J Physiol. 1987 Aug;253(2 Pt 1):C177–C192. doi: 10.1152/ajpcell.1987.253.2.C177. [DOI] [PubMed] [Google Scholar]
- Oakley B., 2nd, Green D. G. Correlation of light-induced changes in retinal extracellular potassium concentration with c-wave of the electroretinogram. J Neurophysiol. 1976 Sep;39(5):1117–1133. doi: 10.1152/jn.1976.39.5.1117. [DOI] [PubMed] [Google Scholar]
- Oakley B., 2nd, Miller S. S., Steinberg R. H. Effect of intracellular potassium upon the electrogenic pump of frog retinal pigment epithelium. J Membr Biol. 1978 Dec 29;44(3-4):281–307. doi: 10.1007/BF01944225. [DOI] [PubMed] [Google Scholar]
- Oakley B., 2nd Potassium and the photoreceptor-dependent pigment epithelial hyperpolarization. J Gen Physiol. 1977 Oct;70(4):405–425. doi: 10.1085/jgp.70.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B., 2nd, Steinberg R. H. Effects of maintained illumination upon [K+]0 in the subretinal space of the frog retina. Vision Res. 1982;22(7):767–773. doi: 10.1016/0042-6989(82)90007-4. [DOI] [PubMed] [Google Scholar]
- Ostwald T. J., Steinberg R. H. Localization of frog retinal pigment epithelium Na+-K+ ATPase. Exp Eye Res. 1980 Sep;31(3):351–360. doi: 10.1016/s0014-4835(80)80043-1. [DOI] [PubMed] [Google Scholar]
- Pautler E. L., Tengerdy C. Transport of acidic amino acids by the bovine pigment epithelium. Exp Eye Res. 1986 Aug;43(2):207–214. doi: 10.1016/s0014-4835(86)80088-4. [DOI] [PubMed] [Google Scholar]
- Shimazaki H., Oakley B., 2nd Reaccumulation of [K+]o in the toad retina during maintained illumination. J Gen Physiol. 1984 Sep;84(3):475–504. doi: 10.1085/jgp.84.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg R. H., Miller S. S., Stern W. H. Initial observations on the isolated retinal pigment epithelium-choroid of the cat. Invest Ophthalmol Vis Sci. 1978 Jul;17(7):675–678. [PubMed] [Google Scholar]
- Steinberg R. H., Oakley B., 2nd, Niemeyer G. Light-evoked changes in [K+]0 in retina of intact cat eye. J Neurophysiol. 1980 Nov;44(5):897–921. doi: 10.1152/jn.1980.44.5.897. [DOI] [PubMed] [Google Scholar]
- Weleber R. G. Fast and slow oscillations of the electro-oculogram in Best's macular dystrophy and retinitis pigmentosa. Arch Ophthalmol. 1989 Apr;107(4):530–537. doi: 10.1001/archopht.1989.01070010544028. [DOI] [PubMed] [Google Scholar]
- Winkler B. S., Giblin F. J. Glutathione oxidation in retina: effects on biochemical and electrical activities. Exp Eye Res. 1983 Feb;36(2):287–297. doi: 10.1016/0014-4835(83)90013-1. [DOI] [PubMed] [Google Scholar]
- la Cour M., Lund-Andersen H., Zeuthen T. Potassium transport of the frog retinal pigment epithelium: autoregulation of potassium activity in the subretinal space. J Physiol. 1986 Jun;375:461–479. doi: 10.1113/jphysiol.1986.sp016128. [DOI] [PMC free article] [PubMed] [Google Scholar]


