Abstract
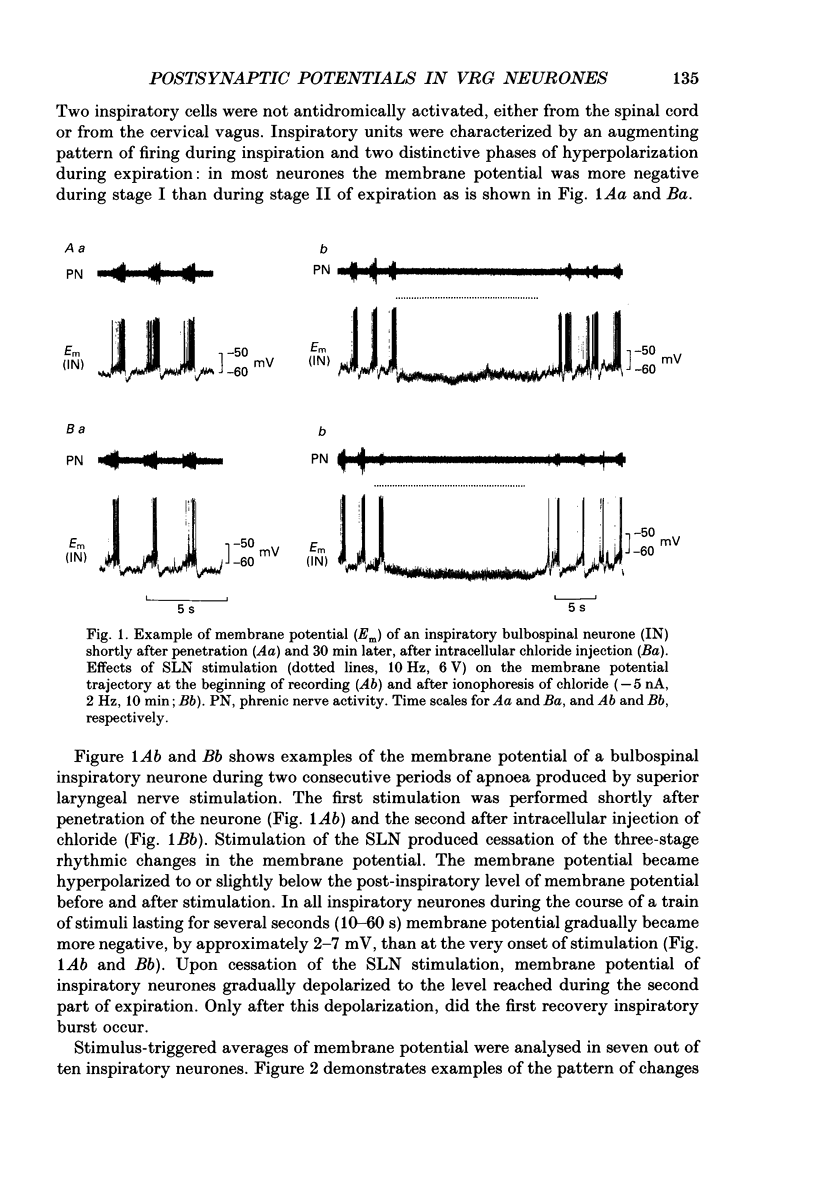
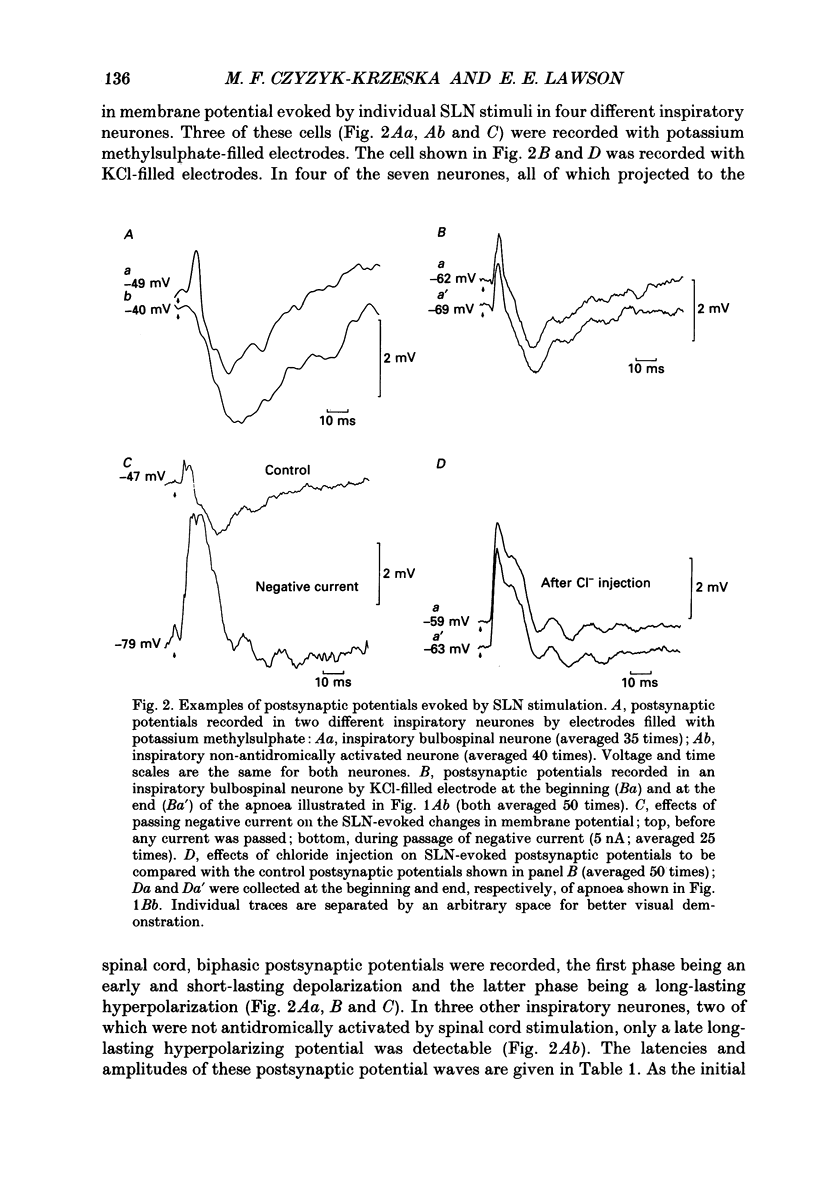
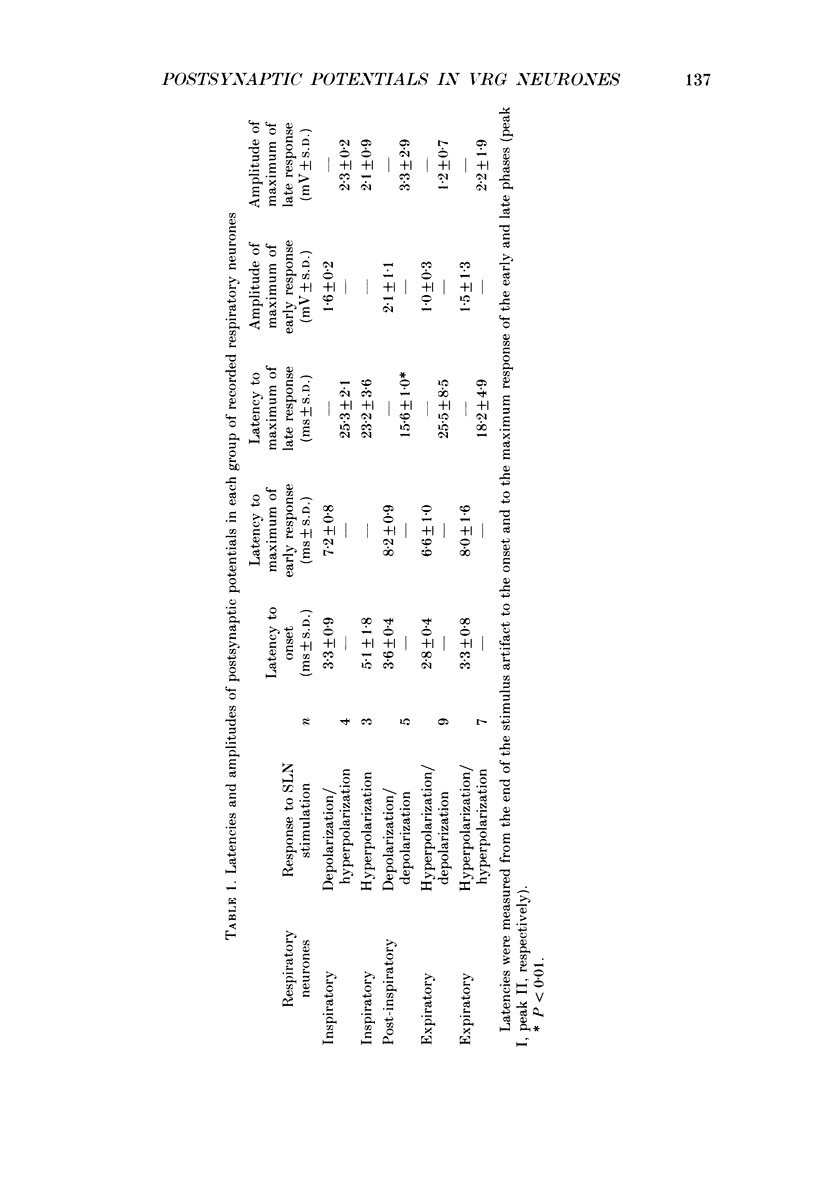
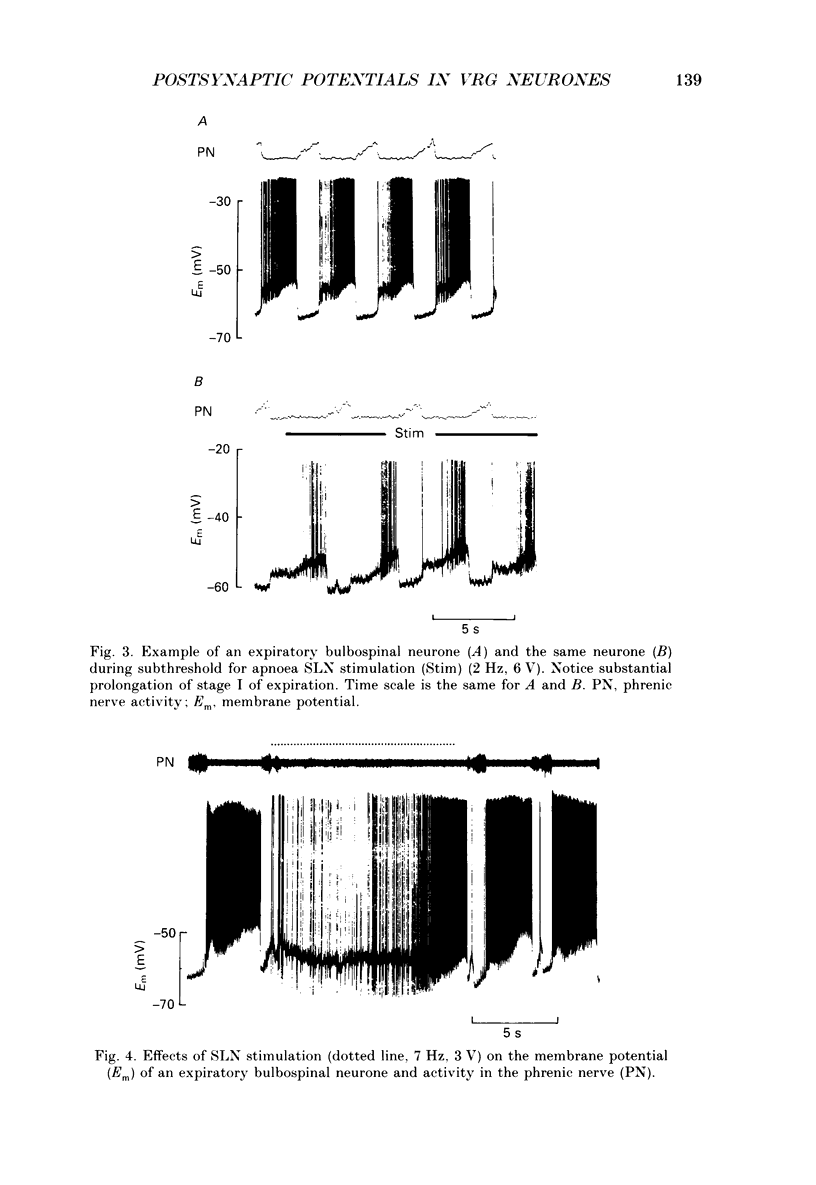
1. Postsynaptic potentials evoked by electrical stimulation of superior laryngeal nerve (SLN) were recorded during SLN-induced apnoea from the respiratory neurones of the ventral respiratory group (VRG) in pentobarbitone-anaesthetized, vagotomized and artificially ventilated newborn piglets (n = 14, 4-7 days old). All recorded inspiratory (n = 10), post-inspiratory (n = 10) and expiratory (n = 20) neurones had a triphasic pattern of membrane potential and were identified for their projections to the spinal cord or cervical vagus nerve. 2. During long-lasting apnoea, induced by SLN stimulation, the membrane potential trajectory of each type of recorded neurone was held at the level corresponding approximately to the membrane potential reached during stage I of expiration. Compound postsynaptic potentials evoked in most respiratory-related neurones had an early short-lasting and a late long-lasting component. 3. Postsynaptic potentials in four out of seven inspiratory neurones, in which postsynaptic potentials were well demonstrated, were characterized by an early depolarization followed by long-lasting hyperpolarization. In three other inspiratory neurones only late hyperpolarization was present. The reversal of the late hyperpolarization by intracellular chloride injection was achieved to a different degree in the early and late portions of late hyperpolarization. 4. Postsynaptic potentials evoked in expiratory neurones were studied in sixteen neurones and displayed two patterns: early hyperpolarization followed by long-lasting hyperpolarization (n = 7, six were not antidromically activated after spinal cord stimulation) or early hyperpolarization followed by late depolarization (n = 9, eight projected to the spinal cord). The early hyperpolarization was readily reversed by chloride injection. The late hyperpolarization was more difficult to reverse and usually the reversal was not completed. 5. Postsynaptic potentials evoked in post-inspiratory neurones showed a pattern of two consecutive phases of depolarization. 6. The present study revealed that during long-lasting apnoea evoked by SLN stimulation each category of VRG respiratory neurones received a temporally synchronized combination of an initial fast input derived reflexly from laryngeal afferents, and of late inputs representing involvement of the whole respiratory network in the response.
Full text
PDF




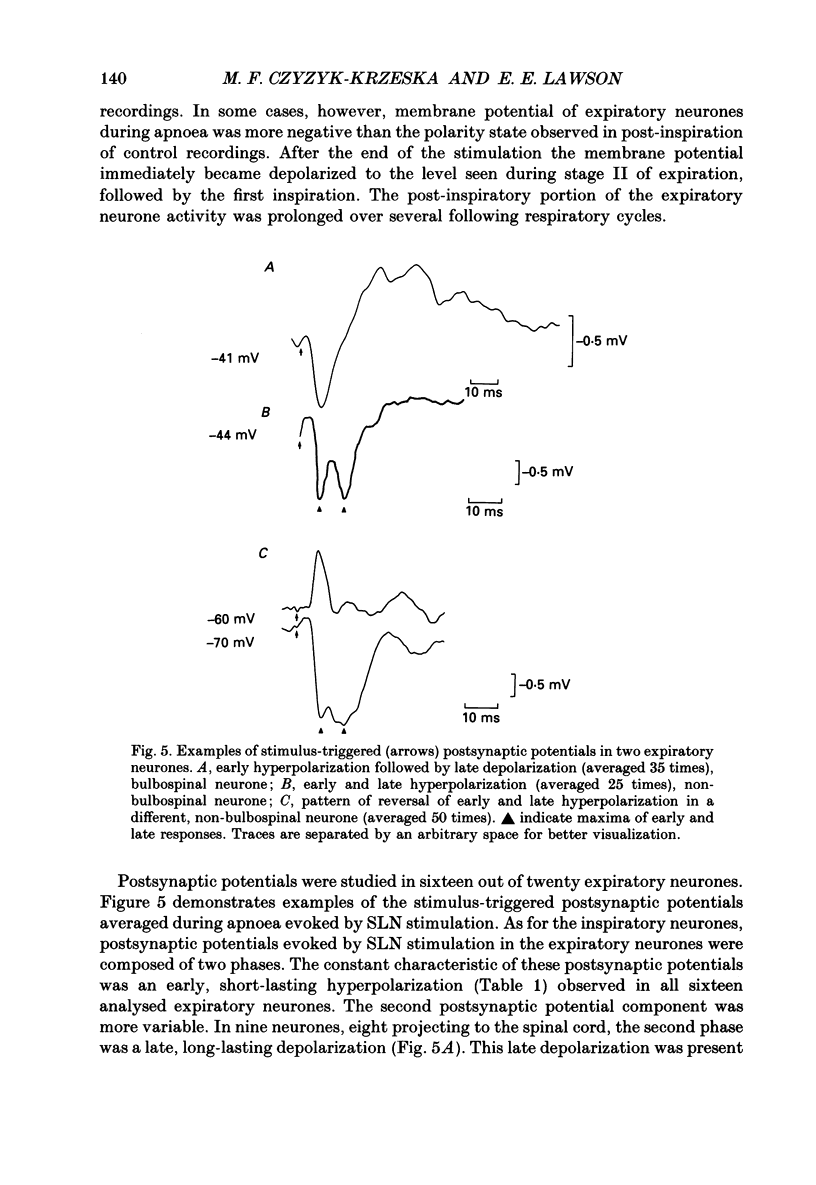
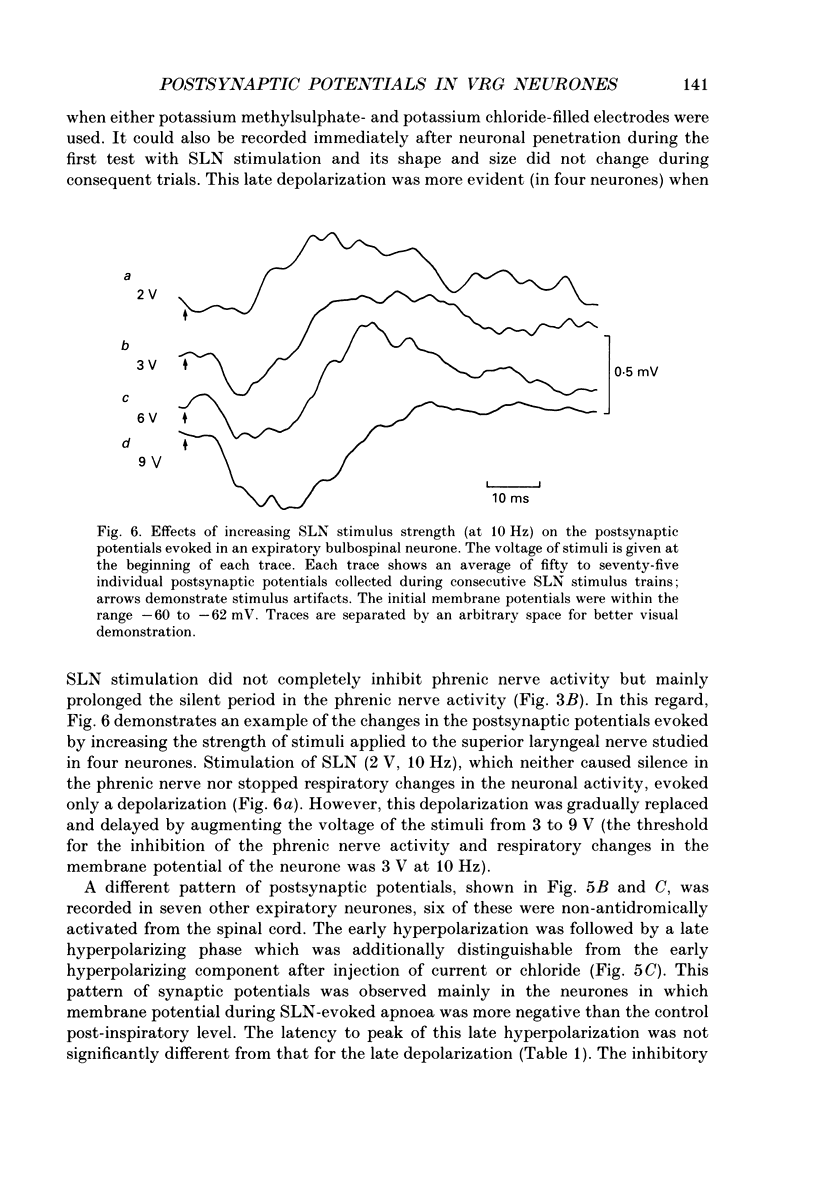
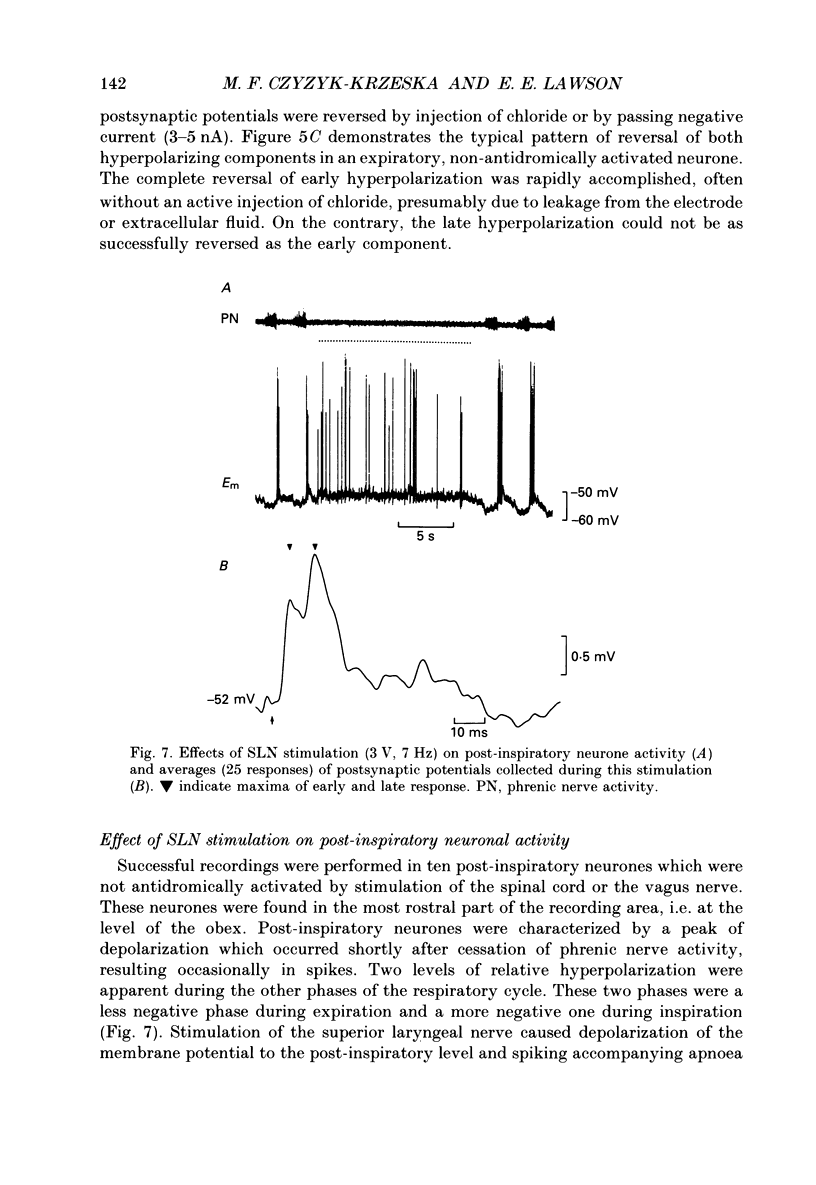





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballantyne D., Richter D. W. The non-uniform character of expiratory synaptic activity in expiratory bulbospinal neurones of the cat. J Physiol. 1986 Jan;370:433–456. doi: 10.1113/jphysiol.1986.sp015943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham M. C., Lipski J., Voss M. D. Synaptic inhibition of phrenic motoneurones evoked by stimulation of the superior laryngeal nerve. Brain Res. 1989 May 8;486(2):391–395. doi: 10.1016/0006-8993(89)90530-1. [DOI] [PubMed] [Google Scholar]
- Berger A. J. Dorsal respiratory group neurons in the medulla of cat: spinal projections, responses to lung inflation and superior laryngeal nerve stimulation. Brain Res. 1977 Oct 28;135(2):231–254. doi: 10.1016/0006-8993(77)91028-9. [DOI] [PubMed] [Google Scholar]
- Berger A. J., Mitchell R. A. Lateralized phrenic nerve responses to stimulating respiratory afferents in the cat. Am J Physiol. 1976 May;230(5):1314–1320. doi: 10.1152/ajplegacy.1976.230.5.1314. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Sampson S. R. An analysis of the inhibition of phrenic motoneurones which occurs on stimulation of some cranial nerve afferents. J Physiol. 1970 Aug;209(2):375–393. doi: 10.1113/jphysiol.1970.sp009170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs D. F., Bartlett D., Jr Chemical specificity of a laryngeal apneic reflex in puppies. J Appl Physiol Respir Environ Exerc Physiol. 1982 Aug;53(2):455–462. doi: 10.1152/jappl.1982.53.2.455. [DOI] [PubMed] [Google Scholar]
- Bongianni F., Corda M., Fontana G., Pantaleo T. Influences of superior laryngeal afferent stimulation on expiratory activity in cats. J Appl Physiol (1985) 1988 Jul;65(1):385–392. doi: 10.1152/jappl.1988.65.1.385. [DOI] [PubMed] [Google Scholar]
- Bystrzycka E. K. Afferent projections to the dorsal and ventral respiratory nuclei in the medulla oblongata of the cat studied by the horseradish peroxidase technique. Brain Res. 1980 Mar 3;185(1):59–66. doi: 10.1016/0006-8993(80)90670-8. [DOI] [PubMed] [Google Scholar]
- Donnelly D. F., Sica A. L., Cohen M. I., Zhang H. Dorsal medullary inspiratory neurons: effects of superior laryngeal afferent stimulation. Brain Res. 1989 Jul 10;491(2):243–252. doi: 10.1016/0006-8993(89)90060-7. [DOI] [PubMed] [Google Scholar]
- Downing S. E., Lee J. C. Laryngeal chemosensitivity: a possible mechanism for sudden infant death. Pediatrics. 1975 May;55(5):640–649. [PubMed] [Google Scholar]
- Duffin J., Aweida D. The propriobulbar respiratory neurons in the cat. Exp Brain Res. 1990;81(2):213–220. doi: 10.1007/BF00228110. [DOI] [PubMed] [Google Scholar]
- Goding G. S., Richardson M. A., Trachy R. E. Laryngeal chemoreflex: anatomic and physiologic study by use of the superior laryngeal nerve in the piglet. Otolaryngol Head Neck Surg. 1987 Jul;97(1):28–38. doi: 10.1177/019459988709700106. [DOI] [PubMed] [Google Scholar]
- Grélot L., Barillot J. C., Bianchi A. L. Pharyngeal motoneurones: respiratory-related activity and responses to laryngeal afferents in the decerebrate cat. Exp Brain Res. 1989;78(2):336–344. doi: 10.1007/BF00228905. [DOI] [PubMed] [Google Scholar]
- Harding R., Johnson P., McClelland M. E. Liquid-sensitive laryngeal receptors in the developing sheep, cat and monkey. J Physiol. 1978 Apr;277:409–422. doi: 10.1113/jphysiol.1978.sp012281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscoe S., Feldman J. L., Cohen M. I. Properties of inspiratory termination by superior laryngeal and vagal stimulation. Respir Physiol. 1979 Apr;36(3):353–366. doi: 10.1016/0034-5687(79)90047-1. [DOI] [PubMed] [Google Scholar]
- Jodkowski J. S., Berger A. J. Influences from laryngeal afferents on expiratory bulbospinal neurons and motoneurons. J Appl Physiol (1985) 1988 Apr;64(4):1337–1345. doi: 10.1152/jappl.1988.64.4.1337. [DOI] [PubMed] [Google Scholar]
- Kalia M., Mesulam M. M. Brain stem projections of sensory and motor components of the vagus complex in the cat: II. Laryngeal, tracheobronchial, pulmonary, cardiac, and gastrointestinal branches. J Comp Neurol. 1980 Sep 15;193(2):467–508. doi: 10.1002/cne.901930211. [DOI] [PubMed] [Google Scholar]
- Kreuter F., Richter D. W., Camerer H., Senekowitsch R. Morphological and electrical description of medullary respiratory neurons of the cat. Pflugers Arch. 1977 Nov 25;372(1):7–16. doi: 10.1007/BF00582200. [DOI] [PubMed] [Google Scholar]
- Lawson E. E. Prolonged central respiratory inhibition following reflex-induced apnea. J Appl Physiol Respir Environ Exerc Physiol. 1981 Apr;50(4):874–879. doi: 10.1152/jappl.1981.50.4.874. [DOI] [PubMed] [Google Scholar]
- Lawson E. E., Richter D. W., Bischoff A. Intracellular recordings of respiratory neurons in the lateral medulla of piglets. J Appl Physiol (1985) 1989 Feb;66(2):983–988. doi: 10.1152/jappl.1989.66.2.983. [DOI] [PubMed] [Google Scholar]
- Loewy A. D., Burton H. Nuclei of the solitary tract: efferent projections to the lower brain stem and spinal cord of the cat. J Comp Neurol. 1978 Sep 15;181(2):421–449. doi: 10.1002/cne.901810211. [DOI] [PubMed] [Google Scholar]
- Pantaleo T., Corda M. Expiration-related neurons in the region of the retrofacial nucleus: vagal and laryngeal inhibitory influences. Brain Res. 1985 Dec 16;359(1-2):343–346. doi: 10.1016/0006-8993(85)91447-7. [DOI] [PubMed] [Google Scholar]
- Remmers J. E., Richter D. W., Ballantyne D., Bainton C. R., Klein J. P. Reflex prolongation of stage I of expiration. Pflugers Arch. 1986 Aug;407(2):190–198. doi: 10.1007/BF00580675. [DOI] [PubMed] [Google Scholar]
- Richter D. W., Ballantyne D., Remmers J. E. The differential organization of medullary post-inspiratory activities. Pflugers Arch. 1987 Nov;410(4-5):420–427. doi: 10.1007/BF00586520. [DOI] [PubMed] [Google Scholar]
- Richter D. W. Generation and maintenance of the respiratory rhythm. J Exp Biol. 1982 Oct;100:93–107. doi: 10.1242/jeb.100.1.93. [DOI] [PubMed] [Google Scholar]
- Sessle B. J. Excitatory and inhibitory inputs to single neurones in the solitary tract nucleus and adjacent reticular formation. Brain Res. 1973 Apr 27;53(2):319–331. doi: 10.1016/0006-8993(73)90217-5. [DOI] [PubMed] [Google Scholar]
- Sica A. L., Cohen M. I., Donnelly D. F., Zhang H. Hypoglossal motoneuron responses to pulmonary and superior laryngeal afferent inputs. Respir Physiol. 1984 Jun;56(3):339–357. doi: 10.1016/0034-5687(84)90069-0. [DOI] [PubMed] [Google Scholar]


