Abstract
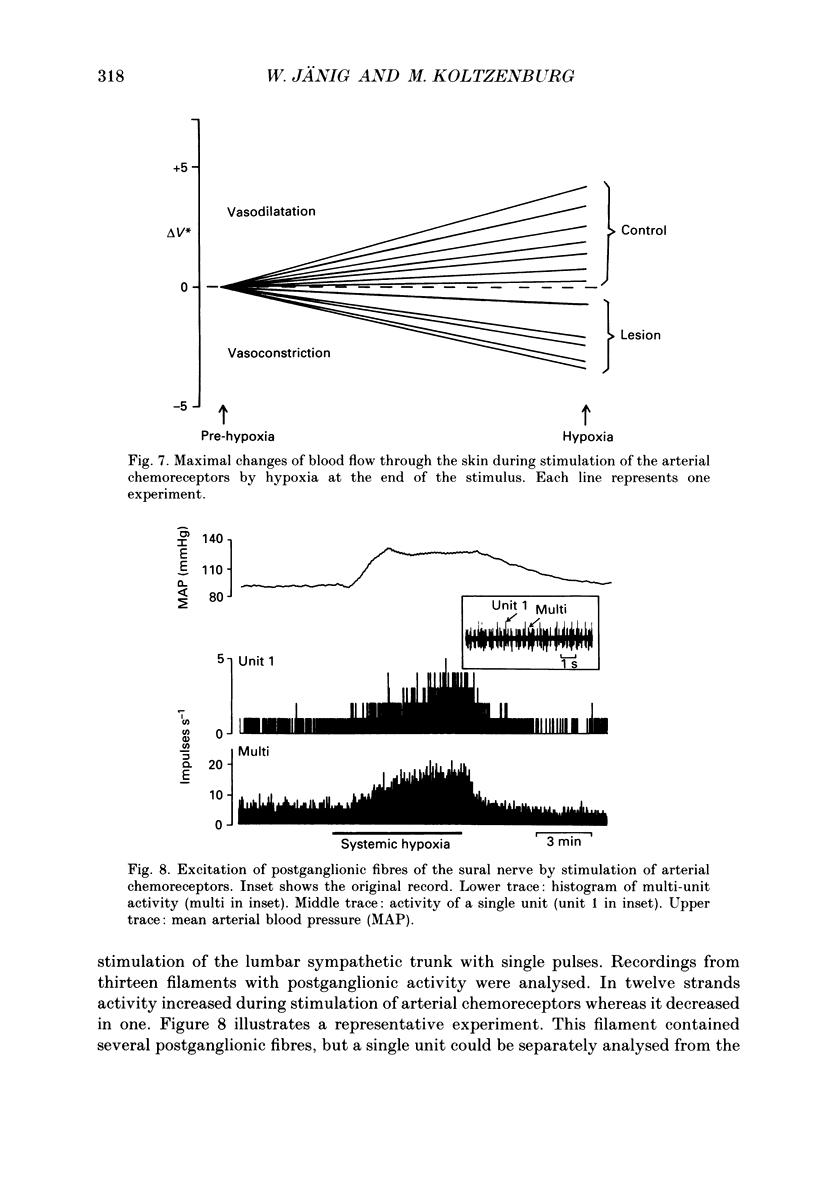
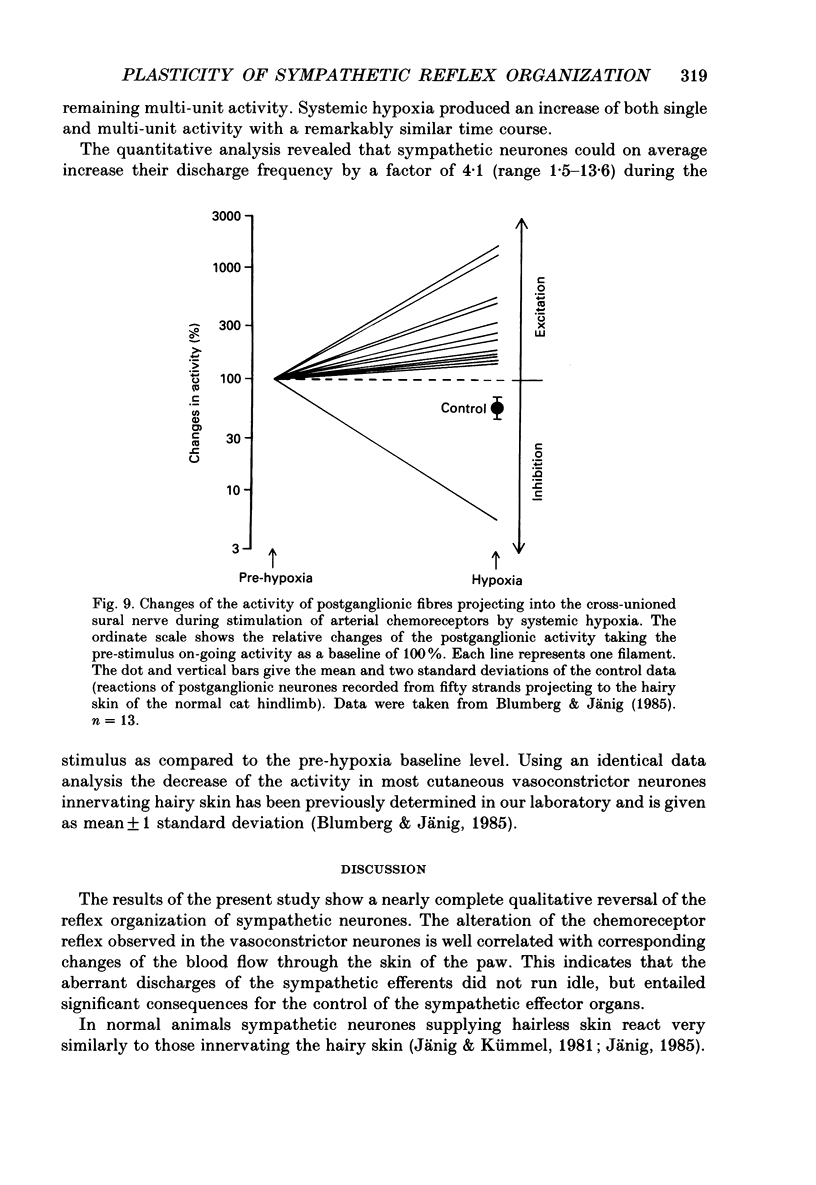
1. The present study has investigated the reflex organization of sympathetic neurones and its control of autonomic effector organs following nerve injury and repair. A well-defined population of vasoconstrictor neurones supplying blood vessels of the hairy skin was forced to innervate a territory that contained some appropriate, but mainly inappropriate autonomic effector organs. For this purpose the central stump of the cut sural nerve was sutured to the peripheral stump of the cut tibial nerve 11-12 months prior to the terminal experiment. 2. The activity of postganglionic sympathetic neurones was recorded from fine strands of the sural nerve proximal to the nerve lesion. Using a laser-Doppler device cutaneous blood flow was measured in the hairless skin of the hindpaw that was now reinnervated by the sural nerve. The results show a qualitative change of the reflex organization of sympathetic neurones following cross-union of these nerves. 3. Stimulation of arterial chemoreceptors by ventilating the animals with a hypoxic gas mixture (8% O2 in N2 for 3-8 min) increased the activity in twelve out of thirteen strands containing postganglionic sympathetic fibres. The increase of sympathetic activity contrasts with results from normal animals where systemic hypoxia causes a reflex decrease of activity in postganglionic fibres of the sural nerve. 4. Reflex changes of sympathetic activity were closely followed by corresponding changes of cutaneous blood flow. Systemic hypoxia produced vasoconstriction in operated animals in contrast to the vasodilatation observed in normal animals. 5. We conclude that the reflex organization of sympathetic neurones can change qualitatively following nerve lesion when sympathetic neurones regenerate and supply inappropriate target tissues. This long-lasting change reflects the plasticity of the autonomic nervous system and can produce a sustained abnormal control of reinnervated autonomic effector organs.
Full text
PDF



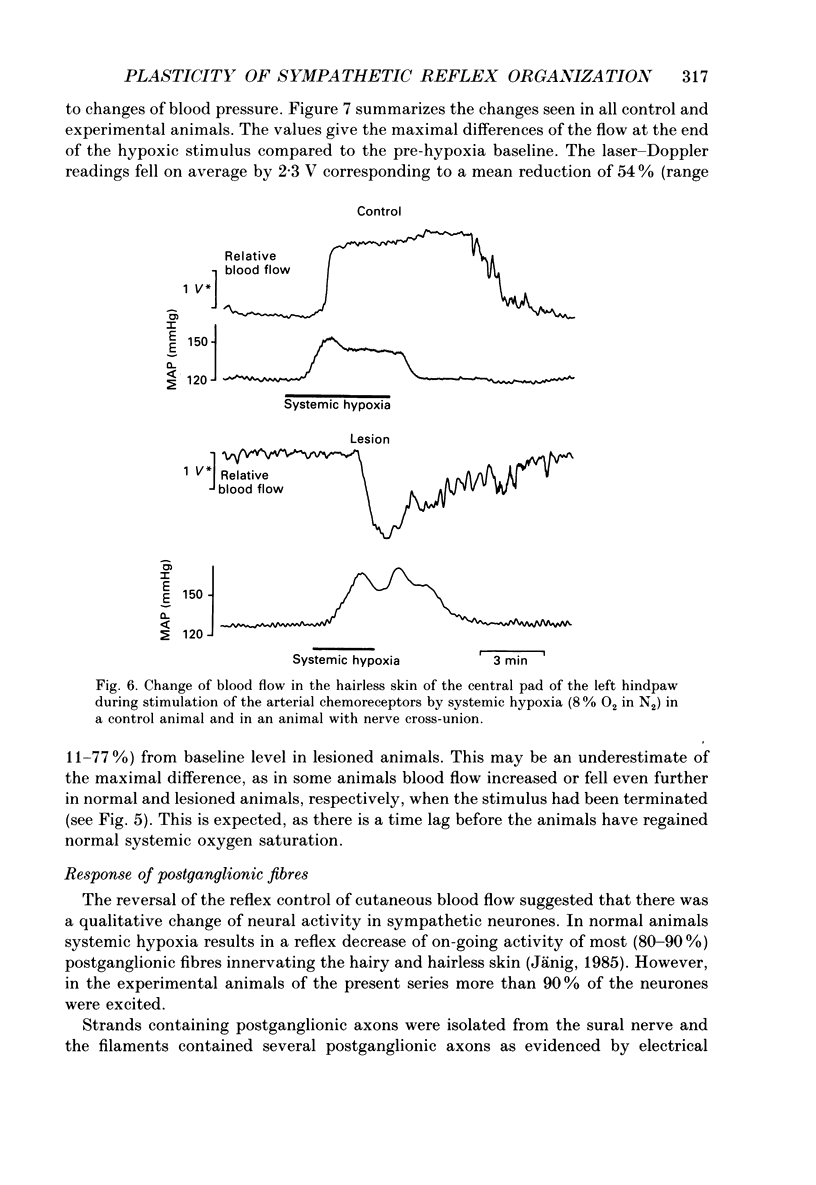




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldskogius H., Arvidsson J., Grant G. The reaction of primary sensory neurons to peripheral nerve injury with particular emphasis on transganglionic changes. Brain Res. 1985 Sep;357(1):27–46. doi: 10.1016/0165-0173(85)90006-2. [DOI] [PubMed] [Google Scholar]
- Banks R. W., Barker D. Specificities of afferents reinnervating cat muscle spindles after nerve section. J Physiol. 1989 Jan;408:345–372. doi: 10.1113/jphysiol.1989.sp017463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg H., Jänig W. Enhancement of resting activity in postganglionic vasoconstrictor neurones following short-lasting repetitive activation of preganglionic axons. Pflugers Arch. 1983 Feb;396(2):89–94. doi: 10.1007/BF00615510. [DOI] [PubMed] [Google Scholar]
- Blumberg H., Jänig W. Reflex patterns in postganglionic vasoconstrictor neurons following chronic nerve lesions. J Auton Nerv Syst. 1985 Oct;14(2):157–180. doi: 10.1016/0165-1838(85)90073-6. [DOI] [PubMed] [Google Scholar]
- Gregor M., Jänig Effects of sytemic hypoxia and hypercapnia on cutaneous and muscle vasoconstrictor neurones to the cat's hindlimb. Pflugers Arch. 1977 Mar 11;368(1-2):71–81. doi: 10.1007/BF01063457. [DOI] [PubMed] [Google Scholar]
- Häbler H. J., Jänig W., Koltzenburg M. Activation of unmyelinated afferents in chronically lesioned nerves by adrenaline and excitation of sympathetic efferents in the cat. Neurosci Lett. 1987 Nov 10;82(1):35–40. doi: 10.1016/0304-3940(87)90167-4. [DOI] [PubMed] [Google Scholar]
- Jänig W., Krauspe R., Wiedersatz G. Transmission of impulses from pre- to postganglionic vasoconstrictor and sudomotor neurons. J Auton Nerv Syst. 1982 Jul;6(1):95–106. doi: 10.1016/0165-1838(82)90026-1. [DOI] [PubMed] [Google Scholar]
- Jänig W., Kümmel H. Organization of the sympathetic innervation supplying the hairless skin of the cat's paw. J Auton Nerv Syst. 1981 Apr;3(2-4):215–230. doi: 10.1016/0165-1838(81)90064-3. [DOI] [PubMed] [Google Scholar]
- Jänig W., Lisney S. J. Small diameter myelinated afferents produce vasodilatation but not plasma extravasation in rat skin. J Physiol. 1989 Aug;415:477–486. doi: 10.1113/jphysiol.1989.sp017732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänig W. Organization of the lumbar sympathetic outflow to skeletal muscle and skin of the cat hindlimb and tail. Rev Physiol Biochem Pharmacol. 1985;102:119–213. doi: 10.1007/BFb0034086. [DOI] [PubMed] [Google Scholar]
- Koerber H. R., Seymour A. W., Mendell L. M. Mismatches between peripheral receptor type and central projections after peripheral nerve regeneration. Neurosci Lett. 1989 Apr 24;99(1-2):67–72. doi: 10.1016/0304-3940(89)90266-8. [DOI] [PubMed] [Google Scholar]
- Langley J. N. On the Regeneration of Pre-Ganglionic and of Post-Ganglionic Visceral Nerve Fibres. J Physiol. 1897 Nov 20;22(3):215–230. doi: 10.1113/jphysiol.1897.sp000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews M. R., Nelson V. H. Detachment of structurally intact nerve endings from chromatolytic neurones of rat superior cervical ganglion during the depression of synaptic transmission induced by post-ganglionic axotomy. J Physiol. 1975 Feb;245(1):91–135. doi: 10.1113/jphysiol.1975.sp010837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon S. B., Gibson S. Peptide expression is altered when afferent nerves reinnervate inappropriate tissue. Neurosci Lett. 1987 Jan 2;73(1):9–15. doi: 10.1016/0304-3940(87)90022-x. [DOI] [PubMed] [Google Scholar]
- McMahon S. B., Moore C. E. Plasticity of primary afferent acid phosphatase expression following rerouting of afferents from muscle to skin in the adult rat. J Comp Neurol. 1988 Aug 1;274(1):1–8. doi: 10.1002/cne.902740102. [DOI] [PubMed] [Google Scholar]
- McMahon S. B., Wall P. D. Changes in spinal cord reflexes after cross-anastomosis of cutaneous and muscle nerves in the adult rat. Nature. 1989 Nov 16;342(6247):272–274. doi: 10.1038/342272a0. [DOI] [PubMed] [Google Scholar]
- Purves D. Functional and structural changes in mammalian sympathetic neurones following interruption of their axons. J Physiol. 1975 Nov;252(2):429–463. doi: 10.1113/jphysiol.1975.sp011151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D., Snider W. D., Voyvodic J. T. Trophic regulation of nerve cell morphology and innervation in the autonomic nervous system. Nature. 1988 Nov 10;336(6195):123–128. doi: 10.1038/336123a0. [DOI] [PubMed] [Google Scholar]
- Wall J. T. Variable organization in cortical maps of the skin as an indication of the lifelong adaptive capacities of circuits in the mammalian brain. Trends Neurosci. 1988 Dec;11(12):549–557. doi: 10.1016/0166-2236(88)90184-1. [DOI] [PubMed] [Google Scholar]
- Wall P. D., Woolf C. J. Muscle but not cutaneous C-afferent input produces prolonged increases in the excitability of the flexion reflex in the rat. J Physiol. 1984 Nov;356:443–458. doi: 10.1113/jphysiol.1984.sp015475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawo H. Changes in the dendritic geometry of mouse superior cervical ganglion cells following postganglionic axotomy. J Neurosci. 1987 Nov;7(11):3703–3711. doi: 10.1523/JNEUROSCI.07-11-03703.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


