Abstract
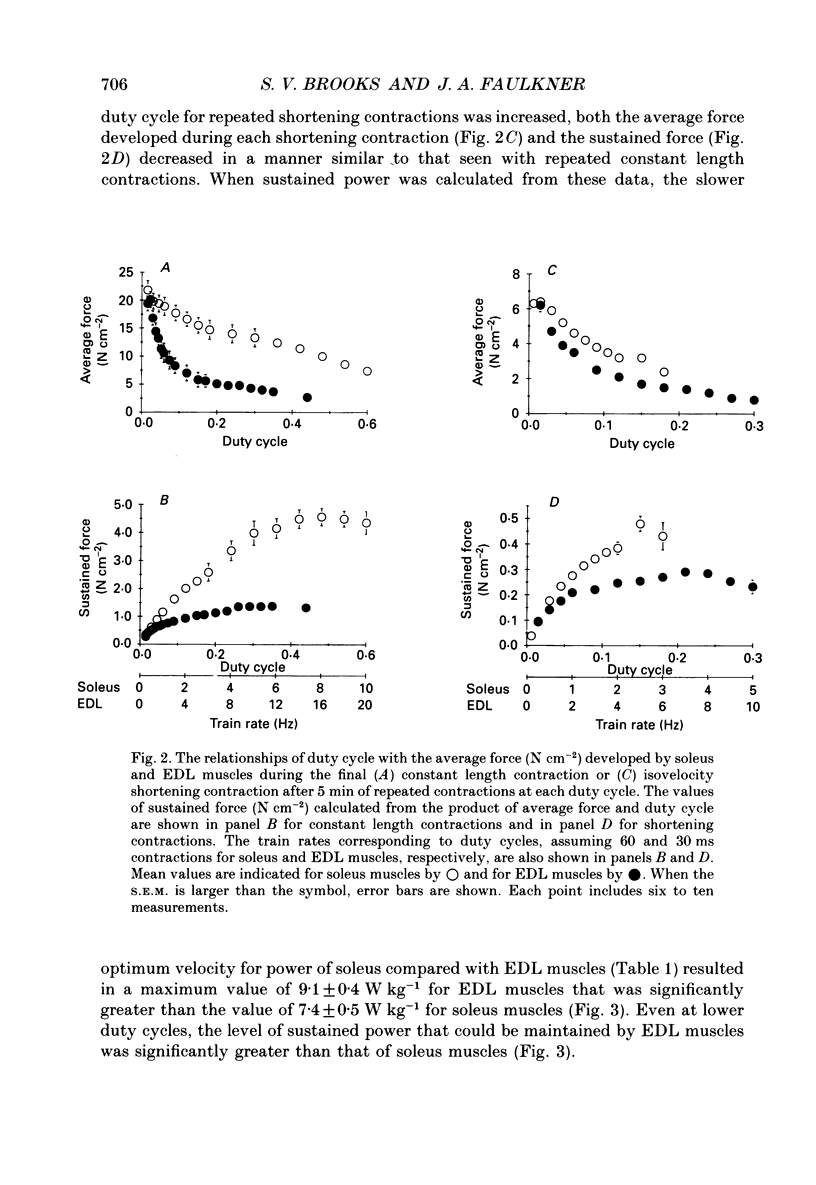
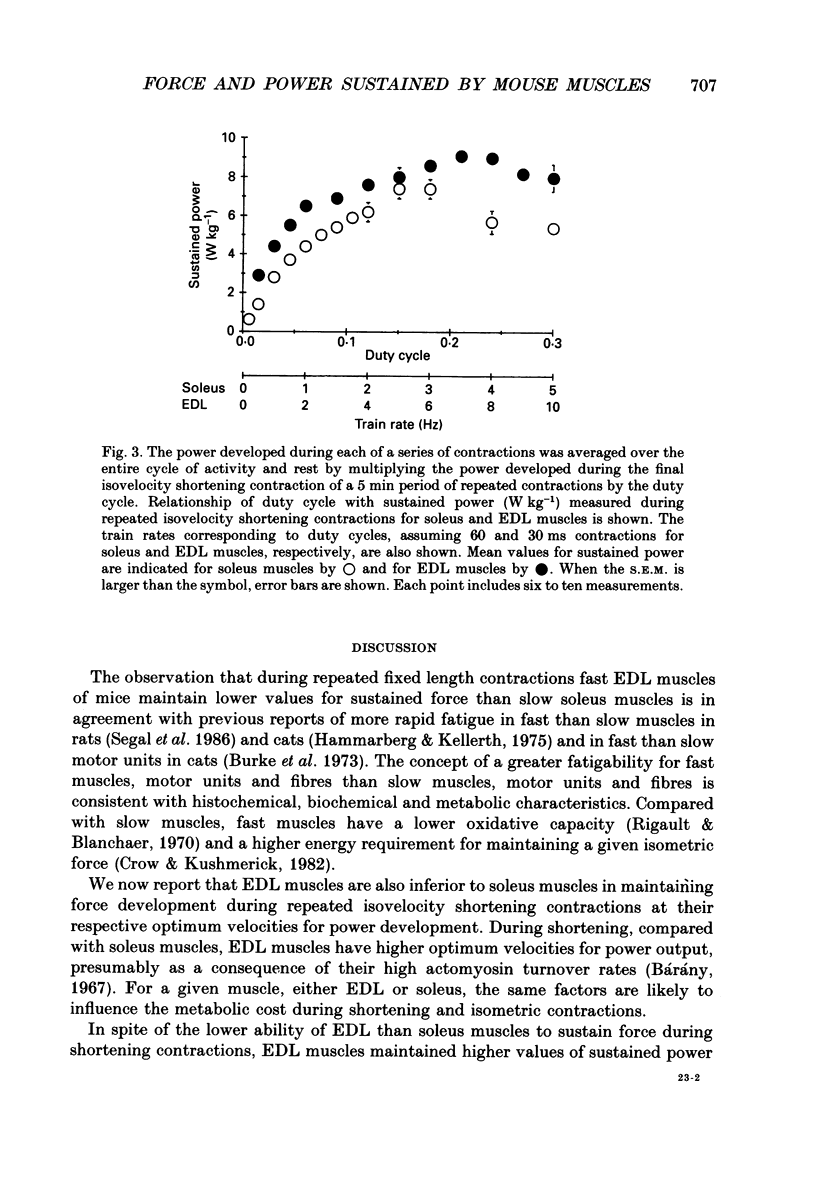
1. The normalized force or power developed during each of a series of repeated contractions was averaged over the entire cycle of activity and rest to provide a measure of performance referred to as sustained force or sustained power. We tested the hypotheses that compared with slow soleus muscles, fast extensor digitorum longus (EDL) muscles would attain lower maximum values of sustained force, but higher maximum values of sustained power. 2. During repeated contractions at a stimulation frequency of 150 Hz, forces and powers of soleus and EDL muscles of mice were determined in situ at 35 degrees C. The train rate of repeated contractions was incremented every 5 min to increase duty cycle until a maximum value for sustained force or power was reached. 3. In one set of repeated contractions, each contraction was preceded by a quick stretch and thereafter muscle length was held constant. The stretch minimized active shortening of muscle fibres. Sustained force was calculated from force at constant length. The maximum sustained force developed by soleus muscles of 4.58 +/- 0.31 N cm-2 (mean +/- S.E.M.) occurred at a duty cycle of 0.48. Compared with soleus muscles, EDL muscles attained a lower (P less than 0.05) maximum value of 1.38 +/- 0.15 N cm-2 at a 0.35 duty cycle. 4. During isovelocity shortening contractions, the maximum value for sustained power developed by soleus muscles of 7.4 +/- 0.5 W kg-1 occurred at a duty cycle of 0.18. Compared with soleus muscles, EDL muscles achieved a significantly greater maximum value of 9.1 +/- 0.4 W kg-1 at a 0.21 duty cycle.
Full text
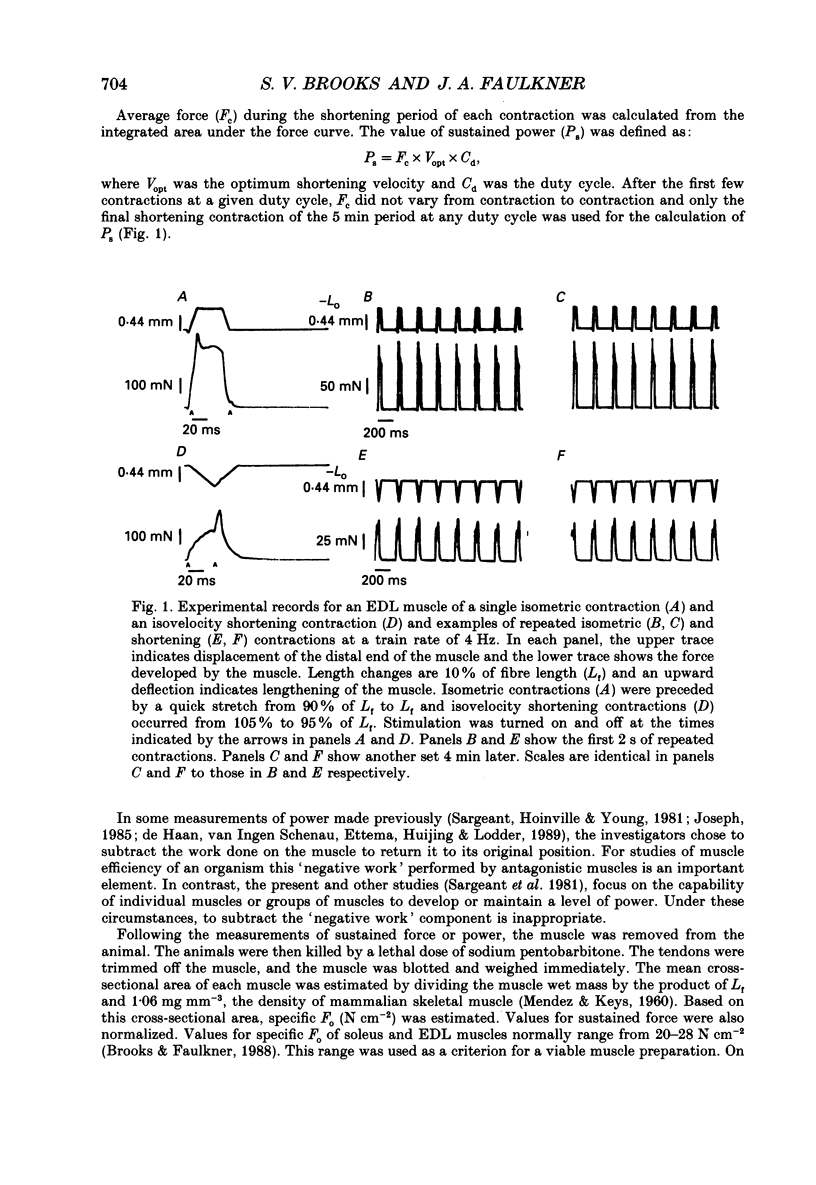
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarado Rigault M. Y., Blanchaer M. C. Respiration and oxidative phosphorylation by mitochondria of red and white skeletal muscle. Can J Biochem. 1970 Jan;48(1):27–32. doi: 10.1139/o70-005. [DOI] [PubMed] [Google Scholar]
- BALKE B., WARE R. W. An experimental study of physical fitness of Air Force personnel. U S Armed Forces Med J. 1959 Jun;10(6):675–688. [PubMed] [Google Scholar]
- Brooks S. V., Faulkner J. A. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol. 1988 Oct;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks S. V., Faulkner J. A., McCubbrey D. A. Power outputs of slow and fast skeletal muscles of mice. J Appl Physiol (1985) 1990 Mar;68(3):1282–1285. doi: 10.1152/jappl.1990.68.3.1282. [DOI] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Tsairis P., Zajac F. E., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973 Nov;234(3):723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLOSE R. DYNAMIC PROPERTIES OF FAST AND SLOW SKELETAL MUSCLES OF THE RAT DURING DEVELOPMENT. J Physiol. 1964 Sep;173:74–95. doi: 10.1113/jphysiol.1964.sp007444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow M. T., Kushmerick M. J. Chemical energetics of slow- and fast-twitch muscles of the mouse. J Gen Physiol. 1982 Jan;79(1):147–166. doi: 10.1085/jgp.79.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Haan A., Van Ingen Schenau G. J., Ettema G. J., Huijing P. A., Lodder M. A. Efficiency of rat medial gastrocnemius muscle in contractions with and without an active prestretch. J Exp Biol. 1989 Jan;141:327–341. doi: 10.1242/jeb.141.1.327. [DOI] [PubMed] [Google Scholar]
- Griffiths R. I. Ultrasound transit time gives direct measurement of muscle fibre length in vivo. J Neurosci Methods. 1987 Oct;21(2-4):159–165. doi: 10.1016/0165-0270(87)90113-0. [DOI] [PubMed] [Google Scholar]
- Hammarberg C., Kellerth J. O. The postnatal development of some twitch and fatigue properties of the ankle flexor and extensor muscles of the cat. Acta Physiol Scand. 1975 Oct;95(2):166–178. doi: 10.1111/j.1748-1716.1975.tb10039.x. [DOI] [PubMed] [Google Scholar]
- Loiselle D. S., Walmsley B. Cost of force development as a function of stimulus rate in rat soleus muscle. Am J Physiol. 1982 Nov;243(5):C242–C246. doi: 10.1152/ajpcell.1982.243.5.C242. [DOI] [PubMed] [Google Scholar]
- Luff A. R. Dynamic properties of the inferior rectus, extensor digitorum longus, diaphragm and soleus muscles of the mouse. J Physiol. 1981;313:161–171. doi: 10.1113/jphysiol.1981.sp013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler M. First-order kinetics of muscle oxygen consumption, and an equivalent proportionality between QO2 and phosphorylcreatine level. Implications for the control of respiration. J Gen Physiol. 1985 Jul;86(1):135–165. doi: 10.1085/jgp.86.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCully K. K., Faulkner J. A. Injury to skeletal muscle fibers of mice following lengthening contractions. J Appl Physiol (1985) 1985 Jul;59(1):119–126. doi: 10.1152/jappl.1985.59.1.119. [DOI] [PubMed] [Google Scholar]
- Sargeant A. J., Hoinville E., Young A. Maximum leg force and power output during short-term dynamic exercise. J Appl Physiol Respir Environ Exerc Physiol. 1981 Nov;51(5):1175–1182. doi: 10.1152/jappl.1981.51.5.1175. [DOI] [PubMed] [Google Scholar]
- Segal S. S., Faulkner J. A., White T. P. Skeletal muscle fatigue in vitro is temperature dependent. J Appl Physiol (1985) 1986 Aug;61(2):660–665. doi: 10.1152/jappl.1986.61.2.660. [DOI] [PubMed] [Google Scholar]
- TABAKIN B. S., HANSON J. S., MERRIAM T. W., Jr, CALDWELL E. J. HEMODYNAMIC RESPONSE OF NORMAL MEN TO GRADED TREADMILL EXERCISE. J Appl Physiol. 1964 May;19:457–464. doi: 10.1152/jappl.1964.19.3.457. [DOI] [PubMed] [Google Scholar]
- Whipp B. J., Ward S. A. Physiological determinants of pulmonary gas exchange kinetics during exercise. Med Sci Sports Exerc. 1990 Feb;22(1):62–71. [PubMed] [Google Scholar]


