Abstract
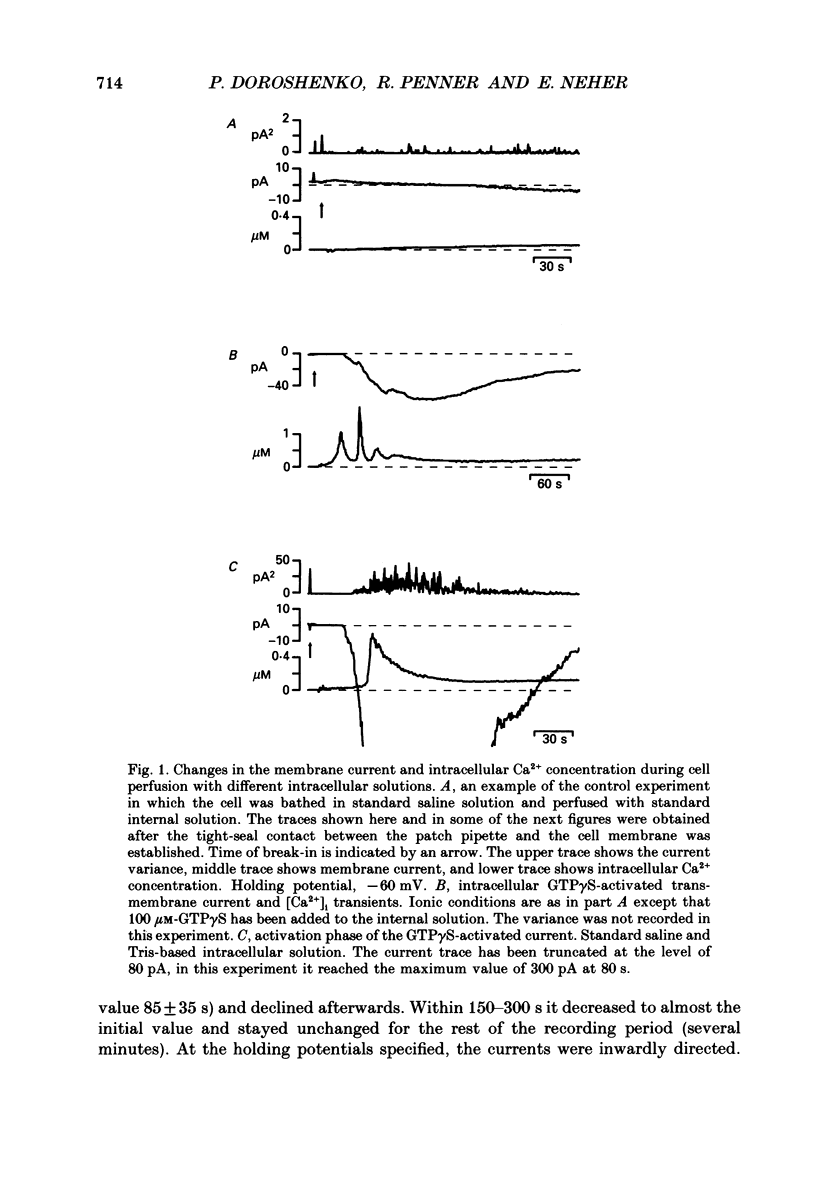
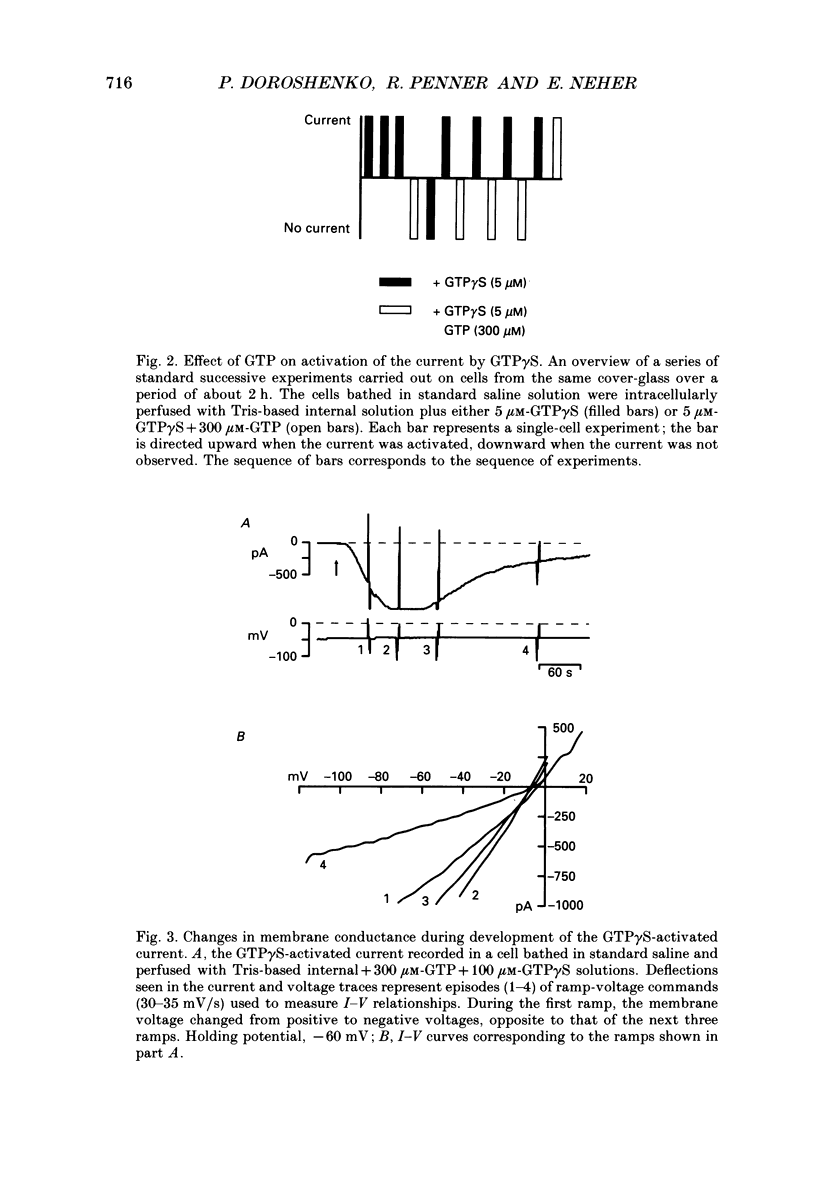
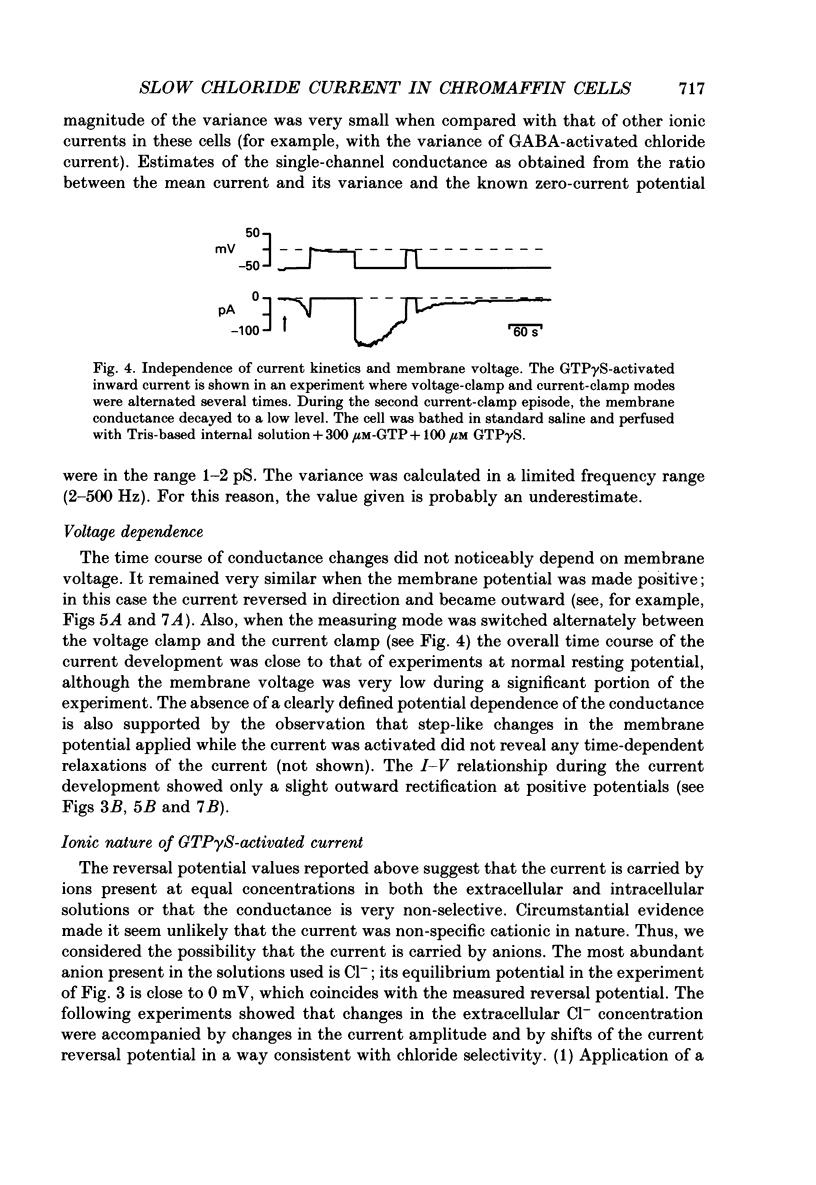
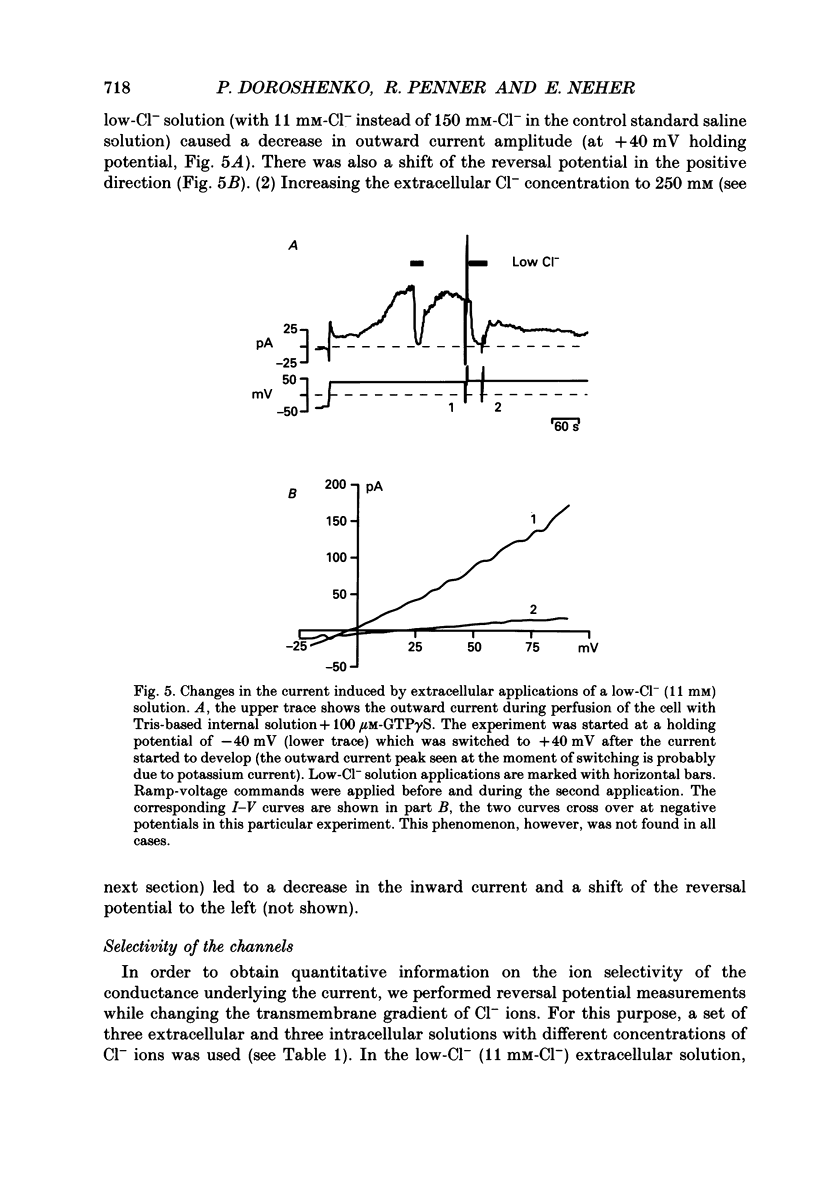
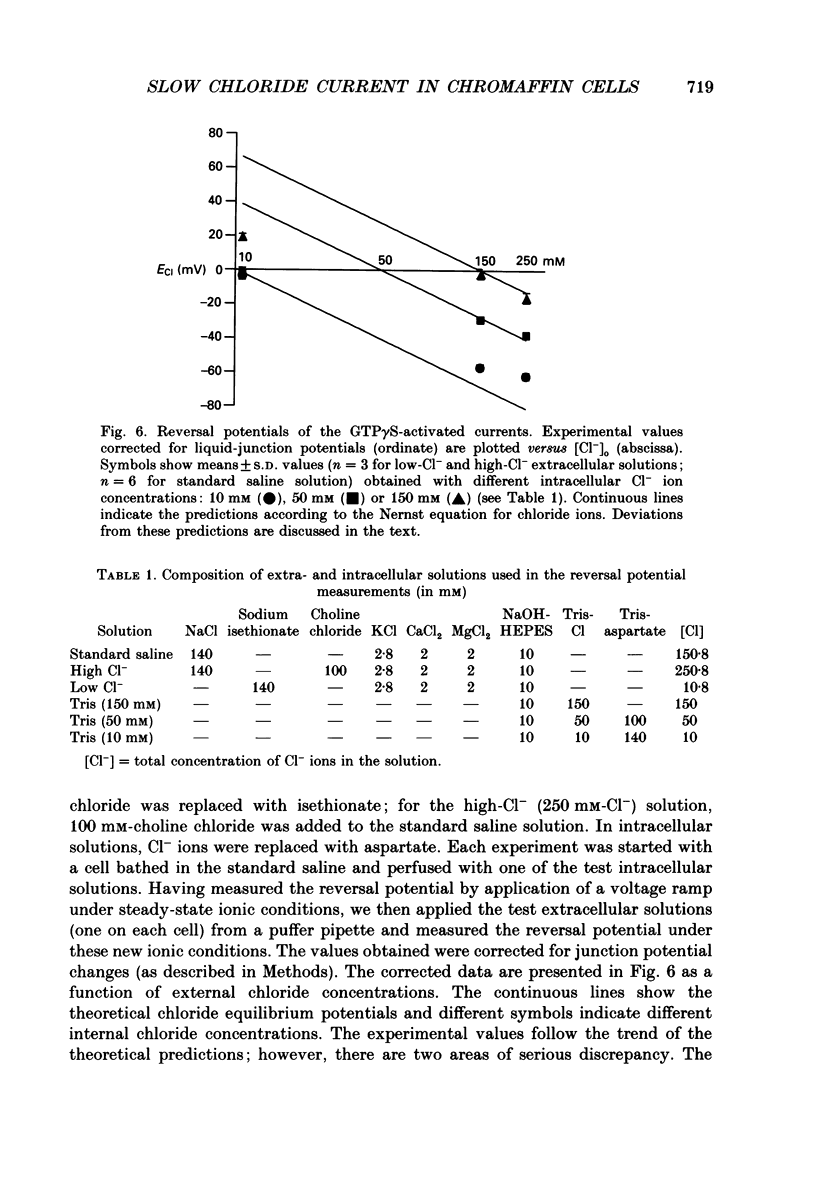
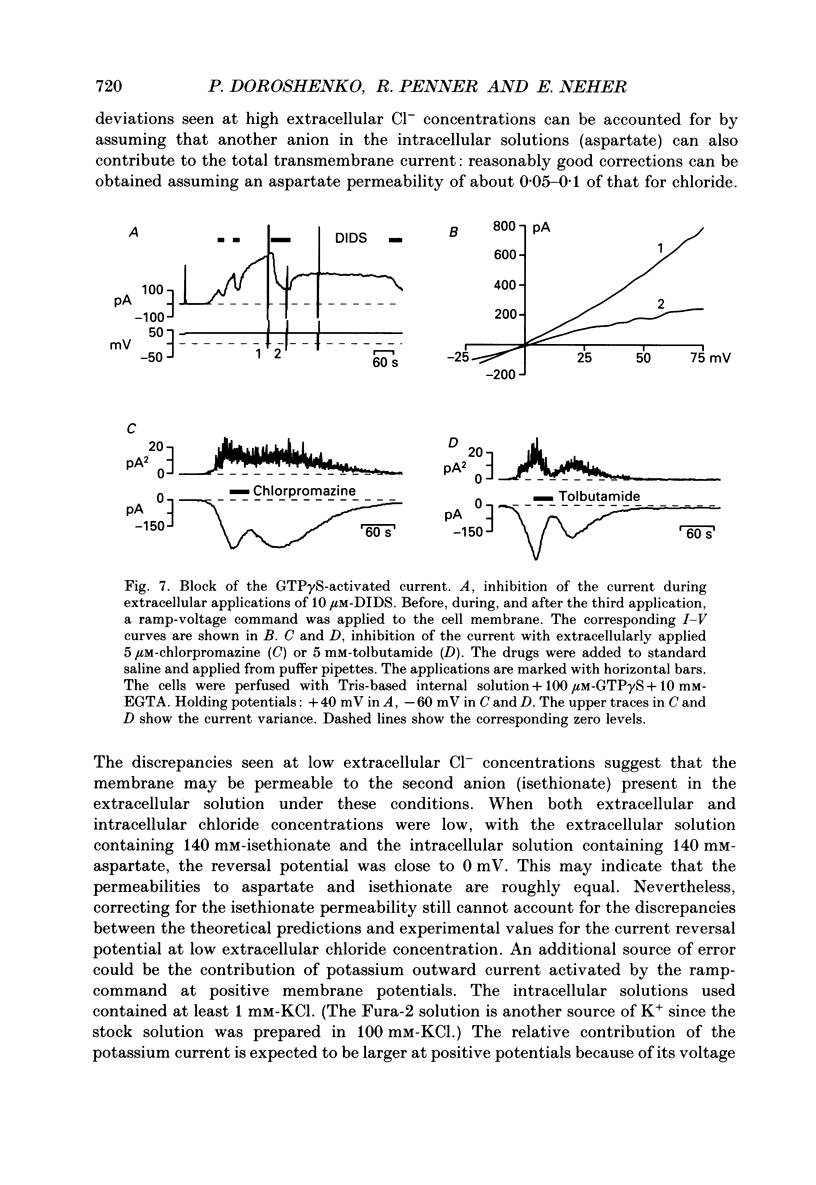
1. The effects of introducing the non-hydrolysable GTP analogue guanosine 5'-O-(3-thiotriphosphate) (GTP gamma S) into perfused bovine chromaffin cells were studied by a combination of the tight-seal whole-cell patch-clamp technique and Fura-2 fluorescence [Ca2+]i measurements. 2. GTP gamma S (5-300 microM) induced a slowly developing transient current (inwardly directed at the holding potential -60 to -70 mV) and [Ca2+]i oscillations. The current activated with a 10-50 s delay after the start of whole-cell dialysis, peaked at 70-120 s and decayed almost to its initial level during the next 150-300 s. Calcium oscillations were observed within the first 100-150 s of cell perfusion. 3. GTP competitively lowered the probability of current activation by GTP gamma S. At low GTP gamma S/GTP ratio (5 and 300 microM, respectively) activation of the current was observed only rarely. 4. The activation of the current was accompanied by an increase in conductance but not by changes in the current reversal potential. The changes in the conductance did not depend on the membrane potential; no time-dependent relaxation of the current was induced by steps in the membrane voltage. 5. The current reversal potential was close to the Cl- equilibrium potential; changes in the extracellular Cl- concentration induced corresponding changes in the current amplitude and shifted its reversal potential. The permeability to larger anions--aspartate, glutamate and isethionate--was about one-tenth of that for chloride. 6. Single-channel conductance, estimated from the ratio of the mean current and its variance, was about 1-2 pS. 7. The current could be reversibly blocked by 4,4'-diisothiocyanatostilbene-2,2'-disulphonate (DIDS, 10 microM), chlorpromazine (5 microM) and tolbutamide (0.5-5 mM). 8. It is suggested that the GTP gamma S-induced increase in the permeability to Cl- ions is due to a G protein-mediated production of an as yet unidentified second messenger.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bommer M., Herz A. Neurotensin affects metabolism of opioid peptides, catecholamines and inositol phospholipids in bovine chromaffin cells. Life Sci. 1989;44(5):327–335. doi: 10.1016/0024-3205(89)90226-9. [DOI] [PubMed] [Google Scholar]
- Bormann J., Clapham D. E. gamma-Aminobutyric acid receptor channels in adrenal chromaffin cells: a patch-clamp study. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2168–2172. doi: 10.1073/pnas.82.7.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt B. L., Hagiwara S., Kidokoro Y., Miyazaki S. Action potentials in the rat chromaffin cell and effects of acetylcholine. J Physiol. 1976 Dec;263(3):417–439. doi: 10.1113/jphysiol.1976.sp011638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitwieser G. E., Szabo G. Mechanism of muscarinic receptor-induced K+ channel activation as revealed by hydrolysis-resistant GTP analogues. J Gen Physiol. 1988 Apr;91(4):469–493. doi: 10.1085/jgp.91.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Birnbaumer L. Direct G protein gating of ion channels. Am J Physiol. 1988 Mar;254(3 Pt 2):H401–H410. doi: 10.1152/ajpheart.1988.254.3.H401. [DOI] [PubMed] [Google Scholar]
- Cahalan M. D., Lewis R. S. Role of potassium and chloride channels in volume regulation by T lymphocytes. Soc Gen Physiol Ser. 1988;43:281–301. [PubMed] [Google Scholar]
- Clapham D. E., Neher E. Substance P reduces acetylcholine-induced currents in isolated bovine chromaffin cells. J Physiol. 1984 Feb;347:255–277. doi: 10.1113/jphysiol.1984.sp015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D. E., Neher E. Trifluoperazine reduces inward ionic currents and secretion by separate mechanisms in bovine chromaffin cells. J Physiol. 1984 Aug;353:541–564. doi: 10.1113/jphysiol.1984.sp015350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin A. C., McGuirk S. M., Scott R. H. An investigation into the mechanisms of inhibition of calcium channel currents in cultured sensory neurones of the rat by guanine nucleotide analogues and (-)-baclofen. Br J Pharmacol. 1989 May;97(1):263–273. doi: 10.1111/j.1476-5381.1989.tb11950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. G., Marty A. Potentiation of muscarinic and alpha-adrenergic responses by an analogue of guanosine 5'-triphosphate. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4099–4103. doi: 10.1073/pnas.83.11.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gögelein H. Chloride channels in epithelia. Biochim Biophys Acta. 1988 Oct 11;947(3):521–547. doi: 10.1016/0304-4157(88)90006-8. [DOI] [PubMed] [Google Scholar]
- Hazama A., Okada Y. Ca2+ sensitivity of volume-regulatory K+ and Cl- channels in cultured human epithelial cells. J Physiol. 1988 Aug;402:687–702. doi: 10.1113/jphysiol.1988.sp017229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A., Neher E. Potassium channels in cultured bovine adrenal chromaffin cells. J Physiol. 1985 Oct;367:117–141. doi: 10.1113/jphysiol.1985.sp015817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G., Neher E., Penner R. Chloride conductance activated by external agonists and internal messengers in rat peritoneal mast cells. J Physiol. 1989 Nov;418:131–144. doi: 10.1113/jphysiol.1989.sp017831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C., White M. M. Dimeric structure of single chloride channels from Torpedo electroplax. Proc Natl Acad Sci U S A. 1984 May;81(9):2772–2775. doi: 10.1073/pnas.81.9.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misbahuddin M., Isosaki M., Houchi H., Oka M. Muscarinic receptor-mediated increase in cytoplasmic free Ca2+ in isolated bovine adrenal medullary cells. Effects of TMB-8 and phorbol ester TPA. FEBS Lett. 1985 Oct 7;190(1):25–28. doi: 10.1016/0014-5793(85)80419-1. [DOI] [PubMed] [Google Scholar]
- Noble E. P., Bommer M., Liebisch D., Herz A. H1-histaminergic activation of catecholamine release by chromaffin cells. Biochem Pharmacol. 1988 Jan 15;37(2):221–228. doi: 10.1016/0006-2952(88)90721-6. [DOI] [PubMed] [Google Scholar]
- O'Sullivan A. J., Burgoyne R. D. A comparison of bradykinin, angiotensin II and muscarinic stimulation of cultured bovine adrenal chromaffin cells. Biosci Rep. 1989 Apr;9(2):243–252. doi: 10.1007/BF01116001. [DOI] [PubMed] [Google Scholar]
- Plevin R., Boarder M. R. Stimulation of formation of inositol phosphates in primary cultures of bovine adrenal chromaffin cells by angiotensin II, histamine, bradykinin, and carbachol. J Neurochem. 1988 Aug;51(2):634–641. doi: 10.1111/j.1471-4159.1988.tb01085.x. [DOI] [PubMed] [Google Scholar]
- Toselli M., Lux H. D. GTP-binding proteins mediate acetylcholine inhibition of voltage dependent calcium channels in hippocampal neurons. Pflugers Arch. 1989 Jan;413(3):319–321. doi: 10.1007/BF00583548. [DOI] [PubMed] [Google Scholar]
- Trube G., Rorsman P., Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflugers Arch. 1986 Nov;407(5):493–499. doi: 10.1007/BF00657506. [DOI] [PubMed] [Google Scholar]


