Abstract
We previously demonstrated that the major immediate early (MIE) proximal enhancer containing one GC box and the TATA box containing promoter are minimal elements required for transcription and viral replication in human fibroblast cells (H. Isomura, T. Tsurumi, M. F. Stinski, J. Virol. 78:12788-12799, 2004). After infection, the level of Sp1 increased while Sp3 remained constant. Here we report that either Sp1 or Sp3 transcription factors bind to the GC boxes located at approximately positions −55 and −75 relative to the transcription start site (+1). Both the Sp1 and Sp3 binding sites have a positive and synergistic effect on the human cytomegalovirus (HCMV) major immediate-early (MIE) promoter. There was little to no change in MIE transcription or viral replication for recombinant viruses with one or the other Sp1 or Sp3 binding site mutated. In contrast, mutation of both the Sp1 and Sp3 binding sites caused inefficient MIE transcription and viral replication. These data indicate that the Sp1 and Sp3 binding sites have a significant role in HCMV replication in human fibroblast cells.
Human cytomegalovirus (HCMV), a member of the betaherpesvirus family, infects most individuals asymptomatically. The virus persists in CD34+ hematopoietic progenitor cells, monocytes, and CD34+-derived dendritic cells from healthy seropositive individuals (22, 36, 37). Reactivation of HCMV is related to a variety of diseases such as pneumonitis, hepatitis, and retinitis (7, 25). The mechanisms underlying maintenance of the latent viral genome and the switch from the latent to lytic forms of infection remain unclear.
The host range of HCMV is narrow in cell culture. The virus replicates productively in terminally differentiated cells such as fibroblasts, epithelial and endothelial cells, and monocyte-derived macrophages (17, 18, 27, 41, 59, 60, 65). The major immediate-early (MIE) genes of HCMV play a key role in determining the efficiency of viral replication. IE1 (UL123) and IE2 (UL122) encode pIE72 and pIE86 proteins important for regulation of subsequent viral gene expression. The pIE72 protein contributes to efficient viral replication at low multiplicity of infection (MOI) (19, 21). The IE86 protein is essential for early viral gene expression and autoregulates transcription of the IE1 and IE2 genes (12, 43, 47, 56).
The region upstream of the HCMV MIE promoter is divided into three regions: the modulator, the unique region, and the enhancer (52, 61). The modulator has no effect on MIE transcription and on viral replication in cell culture (51). The unique region also has no effect on transcription from the MIE promoter, but one or more cis-acting elements repress transcription from the divergent early viral UL127 promoter (39, 45). The enhancer is divided into distal and proximal components (50). Without the distal enhancer the recombinant virus replicates slowly and has a small-plaque phenotype in human fibroblast cells (50). Proximal and distal chimeras of the human and murine CMV enhancers replicate less efficiently at low MOIs and demonstrate the small-plaque phenotype (29). The entire enhancer region of human CMV is required for robust MIE gene expression.
In a previous report, we demonstrated a direct correlation between the extent of the proximal enhancer and the amount of MIE gene transcription and the level of infectious virus replication (30). Deletion of the enhancer from nucleotide position −636 to −39 resulted in no replication in human foreskin fibroblasts (HFFs). In contrast, a recombinant virus with a deletion from position −636 to −67 replicated independently. This recombinant virus contained a single GC box upstream of the MIE promoter that binds the Sp1 transcription factor (30).
The Sp1 family of transcription factors is composed of four proteins (Sp1, Sp2, Sp3, and Sp4) (64). In addition to a highly conserved DNA-binding domain, all four cellular proteins have glutamine-rich activation domains adjacent to serine/threonine-rich stretches in their N-terminal regions. Sp1, Sp2, and Sp3 are ubiquitously expressed, but expression of Sp4 is limited to the brain (63). Sp1 and Sp3 both recognize GC-rich sequences known as GC boxes, while Sp2 recognizes a GT-rich sequence (33). It is generally accepted that Sp1 is involved in the activation of a large number of genes necessary for cellular processes such as cell cycle regulation, chromatin remodeling, and the propagation of methylation-free CpG islands (1, 34, 74). Sp3 has been found to act as a transcriptional activator at Sp1-like sites on many promoters (28, 42, 73).
Yurochko et al. have reported that HCMV infection results in a biphasic increase in Sp1 DNA binding activity during HCMV infection (72). The attachment of HCMV glycoproteins gB and gH to the cell surface receptors is responsible for the first phase induction (70). In addition, the pIE86 protein increases the Sp1 DNA binding activity by possibly freeing up inactive Sp1 (72).
In the proximal enhancer of HCMV, there are two Sp1/Sp3 binding sites (GC boxes) at approximately positions −55 and −75 relative to the transcription start site (+1) (30). We investigated the role of the −55 and −75 GC boxes within the entire MIE enhancer on viral gene expression and replication. The other transcription factor binding sites, such as NF-κB and CREB/ATF, were not mutated. Deletion of one Sp1 or Sp3 binding site did not significantly impair viral gene expression and replication in HFF cells. In contrast, deletion of both Sp1 and Sp3 binding sites had a significant effect on viral gene expression and replication.
MATERIALS AND METHODS
Cells and virus titration.
Primary HFF cells were maintained in Eagle's minimal essential medium supplemented with 10% fetal calf serum (Sigma, St. Louis, Mo.), penicillin (100 U/ml), and streptomycin (100 μg/ml) at 37°C in 5% CO2 as described previously (62).
The virus titers of wild-type (wt) HCMV Towne and recombinant viruses were determined by standard plaque assays on HFF cells as described previously (51). Titers of the recombinant viruses with the mutation of the Sp1 binding sites were normalized to wild-type virus as follows. Viral DNA input was determined by infecting HFF cells in 35- or 60-mm plates in triplicate and harvesting the cells at 4 h postinfection (p.i.) in PCR lysis buffer (10 mM Tris-HCl, pH 8.0, 1 mM EDTA, 0.001% TritonX-100, and 0.001% sodium dodecyl sulfate [SDS]) containing 50 μg/ml proteinase K. After incubation at 55°C for 100 min, the proteinase K was inactivated at 95°C for 10 min. The relative amount of input viral DNA was estimated by real-time PCR using HCMV gB primers and probes as described previously (30).
To estimate viral plaque size, plaques were generated from wt or recombinant virus at an MOI of 0.01, and agar was overlaid 3 days p.i. Plaques were measured with an inverted direct scope (Model SZ; Olympus, Tokyo, Japan) for minimum and maximum length, and the results are represented as the average of 10 plaques.
Enzymes.
Restriction endonucleases and calf intestinal alkaline phosphatase were purchased from New England Biolabs Inc. (Beverly, Ma.). High fidelity and expanded high fidelity Taq DNA polymerases were purchased from Invitrogen (Carlsbad, Calif.) and Roche (Mannheim, Germany), respectively, and RNasin and RNase-free DNase were purchased from Promega (Madison, Wis.) and Takara (Tokyo, Japan), respectively. The enzymes were used according to the manufacturers' instructions.
Plasmid construction.
Plasmid pCAT3-Basic vector purchased from Promega has multiple cloning sites upstream of the chloramphenicol acetyltransferase (CAT) gene. Sequences including only the MIE promoter (from position −48 to +53 relative to the transcription start site of +1), the MIE promoter with Sp1(−55) (from position −58 to +53), the MIE promoter with Sp1(−55) and Sp1(−75) (from −77 to +53), or the MIE promoter with mutated Sp1(−55) and Sp1(−75) were amplified by PCR. Plasmid pKS+MIE-583/+78 containing the HCMV Towne DNA from position −583 to +78 of the MIE enhancer and promoter was used as a template. The BglIISp1R reverse primer was used with either the NheITATAF, NheISp1(−55)F, NheISp1(−75)F, or the NheImutSp1(−55)F primer. The sequences of the primers were as follows: BglIISpIR, 5′-GGAAGATCTCGGTGTCTTCTATGGAGGTCAAAACAGCG-3′; NheITATAF, 5′-CTAGCTAGCGGCGTGTACGGTGGGAGGTCTATATAAGC-3′; NheISpI(−55)F, 5′-CTAGCTAGCATGGGCGGTAGGCGTGTACGGT-3′; NheISpI(−75)F, 5′-CTAGCTAGCCCCGCCCCGTTGACGCAAATG-3′; and NheImutSpI(−55)F, 5′-CTAGCTAGCCCCGCCCCGTTGACGCAAATGgatccTAGGCGTGTACG-3′. The amplified products were confirmed by DNA sequencing (Aichi Cancer Center Research Institute Central Facility), digested by restriction endonucleases BglII and NheI, and cloned into the pCAT3-Basic vector at the corresponding restriction endonuclease sites.
CAT assay.
All transfections were in triplicate on 35-mm-diameter plates of HFF cells using Lipofectamine and Plus reagent (Invitrogen) according to the manufacturer's instructions. HFF cells were transfected with 2 μg of each expression plasmid and harvested 72 h posttransfection. Cell lysates were then prepared and subjected to CAT assays as described previously (40). Acetylated and unacetylated [14C]chloramphenicol (Amersham Pharmacia Biotech, Piscataway, N.J.) were separated by thin-layer chromatography in a chloroform-methanol (95:5) solvent. Signal intensity was quantitated with an Image guider (BAS 2500; Fujifilm, Tokyo, Japan) and the percentage of the conversion of unacetylated [14C]chloramphenicol to the acetylated form was calculated. The relative CAT activity of each reporter plasmid was determined based on that of pCAT TATA.
EMSA.
All probes and competitor DNAs were purchased from Invitrogen. The DNA sequences for Sp1(−55 and −75), Sp1(−55), mutSp1(−55), Sp1(−75) and mutSp1(−75) were as follows: Sp1(−55 and −75), 5′-GTCGTAATAACCCCGCCCCGTTGACGCAAATGGGCGGTAGGCGTGTACGGTGGGA-3′ (sense) and 5′-CCTCCCACCGTACACGCCTACCGCCCATTTGCGTCAACGGGGCGGGGTTATTACGAC-3′ (antisense); Sp1(−55), 5′-TGACGCAAATGGGCGGTAGGCGTGT-3′ (sense) and 5′-CGTACACGCCTACCGCCCATTTGC-3′ (antisense); mutSp1(−55), 5′-TGACGCAAATtttCttTAGGCGTGT-3′ (sense) and 5′-CGTACACGCCTAaaGaaaATTTGC-3′ (antisense); Sp1(−75), 5′-GTCGTAATAACCCCGCCCCGTTGAC-3′ (sense) and 5′-TGCGTCAACGGGGCGGGGTTATTAC-3′ (antisense); and mutSp1(−75), 5′-GTCGTAATAAttttGttttGTTGAC-3′ (sense) and 5′-TGCGTCAACaaaaCaaaaTTATTAC-3′ (antisense). Lowercase letters indicate mutant bases. Equal molar ratios of each pair of sense and antisense oligonucleotides were mixed, denatured at 95°C, and annealed by cooling gradually to room temperature (RT). 32P-labeled probes were prepared by 3′ end labeling using the Klenow fragment of Escherichia coli DNA polymerase I and [32P]dGTP (Amersham). Unincorporated deoxynucleoside triphosphates were removed with Chromaspin TE-10 columns (Clontech, Palo Alto, Calif.). An electrophoretic mobility shift assay (EMSA) with nuclear extracts was performed essentially as described (26) with minor modifications.
In antibody supershift experiments, 2-μg aliquots of HeLa nuclear extract were preincubated with 2 μg of poly(dI-dC) in buffer I (20 mM HEPES, pH 7.9, 6.25 mM MgCl2, 0.5 mM EDTA, 0.5 mM dithiothreitol, 0.01% Nonidet P-40, and 9% glycerol) at RT for 15 min. Then, 10 μg of anti-Sp1 and/or anti-Sp3 polyclonal antibodies or control immunoglobulin G (IgG; Zymed, San Francisco, Calif.) was added to the reaction mixture (total volume of 20 μl) at RT for 30 min before 176 fmol of the DNA probe (50,000 cpm) was added. The reaction mixture was allowed to further incubate at RT for 15 min, and the DNA-protein complexes were separated from free probe by electrophoresis in a 5% nondenaturing polyacrylamide gel in 0.5× TAE (20 mM Tris-acetate, pH 7.2, 1.0 mM EDTA) buffer at 4°C. Gels were dried and exposed to Hyperfilm MP (Amersham).
For competition assays (total volume of 20 μl), 2 μg of nuclear extract from HeLa cells was preincubated with the indicated excess molar ratio of nonradioactive competitor double-stranded DNA (dsDNA) and 2 μg of poly(dI-dC) in buffer I at RT for 15 min. Then, 176 fmol (as molecules) of radioactive probe was added to the reaction mixture and incubated further for 15 min at RT. Electrophoresis was performed as described above.
Western blot analysis.
Cells were harvested at the indicated times postinfection, washed with phosphate-buffered saline, and treated with lysis buffer (0.02% SDS, 0.5% Triton X-100, 300 mM NaCl, 20 mM Tris-HCl [pH 7.6], 1 mM EDTA, 1 mM dithiothreitol, 10 μg/ml leupeptin, 5 μg/ml pepstatin A) for 20 min on ice. Samples were centrifuged at 15,000 rpm for 10 min at 4°C, and the clarified cell extracts were measured for protein concentrations using a Bio-Rad protein assay kit. Twenty-microgram aliquots of proteins were loaded in each lane for SDS-10% polyacrylamide gel electrophoresis and then transferred to polyvinylidene difluoride membranes, washed with blotting buffer (1× phosphate-buffered saline containing 0.1% Tween 20), blocked for 60 min in blotting buffer containing 10% low-fat powdered milk, and washed once with blotting buffer. The blots were incubated with primary antibodies at RT for 60 min in blotting buffer containing 5% low-fat powdered milk. They were then further washed in blotting buffer and incubated with horseradish peroxidase-conjugated secondary antibody at room temperature for 60 min. The target proteins were detected with an enhanced chemiluminescence detection system (Amersham). Images were processed by LumiVision PRO (Aisin/Taitec Inc.) with a cooled charge-coupled-device camera and assembled in an Apple G4 computer using Adobe Photoshop 5.0.
Antibodies.
Anti-Sp1 and anti-Sp3 polyclonal antibodies, sc-59 and sc-644, were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). The monoclonal antibodies NEA-9221 against the pIE72 and pIE86 proteins were from Perkin Elmer (Boston, MA). The polyclonal antibody MAB374 against p36 protein encoded by cellular GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was from Chemicon (Temucula, CA).
Mutagenesis of HCMV BAC DNA.
A rapid homologous recombination system in E. coli expressing bacteriophage lambda recombination proteins, exo, beta, and gam (provided by D. Court, National Institutes of Health, Bethesda, MD), was employed as described previously (15). Bacterial artificial chromosome (BAC) DNA of human CMV Towne was obtained from F. Liu (14). Double-stranded DNAs for recombination contained a kanamycin resistance gene flanked by a 34-bp minimal Flp recognition target (FRT) site (5′-GAAGTTCCTATTCTCTAGAAAGTATAGGAACTTC-3′) and from 50 to 70 bp of the homologous viral DNA sequence. To generate deletions of each or both GC boxes located at approximately positions −55 and −75 relative to the start site of +1, primer pairs of BACdelSp1(−55)R and BACdelSp1(−55)F, BACdelSp1(−75)R and BACdelSp1(−75)F, and BACdelSp1(−55)R and BACdelSp1(−75)F were used. Control recombinant viruses with the FRT sequence, the wt+FRT-1, and wt+FRT-2 were constructed using primer pairs of BAC control(−75)F and BACdelSp1(−75)R or BACdelSp1(−75)F and BAC control(−75)R. The sequences of the primers were as follows: BACdelSp1(−55)R, 5′-ACAGCGTGGATGGCGTCTCCAGGCGATCTGACGGTTCACTAAACGAGCTCTGCTTATATAGACCTCCCACGAAGTTCCTATACTTTCTAGAGAATAGGAACTTCGCCAGTGTTACAACCA-3′; BACdelSp1(−55)F, 5′-GAGTTTGTTTTGGCACCAAAATCAACGGGACTTTCCAAAATGTCGTAATAACCCCGCCCCGTTGACGCAAGAAGTTCCTATTCTCTAGAAAGTATAGGAACTTCCGATTTATTCAAC-3′; BACdelSp1(−75)R, 5′-GACGGTTCACTAAACGAGCTCTGCTTATATAGACCTCCCACCGTACACGCCTACCGCCCATTTGCGTCAAGAAGTTCCTATACTTTCTAGAGAATAGGAACTTCGCCAGTGTTACAACCA-3′; BACdelSp1(−75)F, 5′-ACCCCATTGACGTCAATGGGAGTTTGTTTTGGCACCAAAATCAACGGGACTTTCCAAAATGTCGTAATAAGAAGTTCCTATTCTCTAGAAAGTATAGGAACTTCCGATTTATTCAAC-3′; BACcontrol(−75)F, 5′-TTTGTTTTGGCACCAAAATCAACGGGACTTTCCAAAATGTCGTAATAACCCCGCCCCGGAAGTTCCTATTCTCTAGAAAGTATAGGAACTTCAACTCAGCAAAAGTTCGATTTATTCAAC-3′; and BACcontrol(−75)R, 5′-CTGCTTATATAGACCTCCCACCGTACACGCCTACCGCCCATTTGCGTCAACGGGGCGGGGGAAGTTCCTATACTTTCTAGAGAATAGGAACTTCTAATGCTCTGCCAGTGTTACAACCA-3′. Amplification by PCR was performed as follows: 1 cycle of denaturation at 94°C for 2 min; 40 cycles of denaturation at 94°C for 15 s, annealing at 55°C for 30 s, and extension at 72°C for 5 min; 1 cycle of extension at 72°C for 7 min or at 94°C for 2 min; 30 cycles at 94°C for 2 min, 55°C for 2 min, and 72°C for 2 min; and 1 cycle at 72°C for 7 min. To remove residual template DNA, the PCR products were digested with DpnI at 37°C for l.5 h. The DNAs were extracted with phenol-chloroform and precipitated with 95% ethanol. Approximately 100 ng of each DNA fragment was subjected to electroporation into competent E. coli DY380 containing HCMV Towne-BAC DNA. Electroporation was performed using a Bio-Rad Gene Pulser III (2.5 kV, 200 Ω, and 25 μF) following the suggestions of W. Dunn and F. Liu (14). Bacteriophage-encoded recombination proteins for homologous recombination were induced at 42°C for 15 min as described previously (15).
Excision of the kanamycin resistance gene.
To delete the kanamycin resistance gene, recombinant HCMV BAC DNA was transformed into E. coli DH10B. Plasmid pCP20 (provided by G. Hahn, Max von Pettenkofer Institute, Munich, Germany) (23) was transformed into DH10B containing the recombinant HCMV BAC DNA, and HCMV BAC DNA without kanamycin was selected on LB plates containing ampicillin and chloramphenicol.
Recombinant virus isolation.
HFF cells were transfected with either 5 or 10 μg of each recombinant BAC DNA in the presence of 1 μg of plasmid pSVpp71 by the calcium phosphate precipitation method of Graham and Van der Eb (20). After 5 to 20 days, viral plaques appeared. Five to seven days after 100% cytopathic effect, the extracellular fluid containing free virus was stored at −80°C in 50% fetal calf serum until used. Stored viruses were either undiluted or diluted 1:10 and used for infection of HFF cells.
PCR analysis.
PCR analysis was performed using the primer pair HCMVF (5′-CCCGGTGTCTTCTATGGAGGT-3′) and HCMVUL127R (5′-GGTTATATAGCATAAATCAATATTGGCTATTGG-3′). The PCR cycling program was 1 cycle of denaturing at 94°C for 2 min; 30 cycles of denaturing at 94°C for 15 sec, annealing at 55°C for 30 s, and elongation at 72°C for 1 min 30 s; and 1 cycle of elongation at 72°C for 7 min. PCR products were cloned into a TA cloning vector and sequenced to confirm the recombination and excision.
Southern blot analysis.
Recombinant BAC DNAs were purified using a NucleoBond kit (Macherey-Nagel Duren, Germany), digested with restriction endonucleases BlpI and XhoI and subjected to 1.0% agarose gel electrophoresis as described previously (69). Southern blot analysis was performed as described previously (51). DNA fragments (EagI-SpeI) of pKS-583/+78 (see Fig. 4) were labeled using the Megaprime DNA labeling system (Amersham Pharmacia Biotech) and [32P]dCTP (Amersham Pharmacia Biotech).
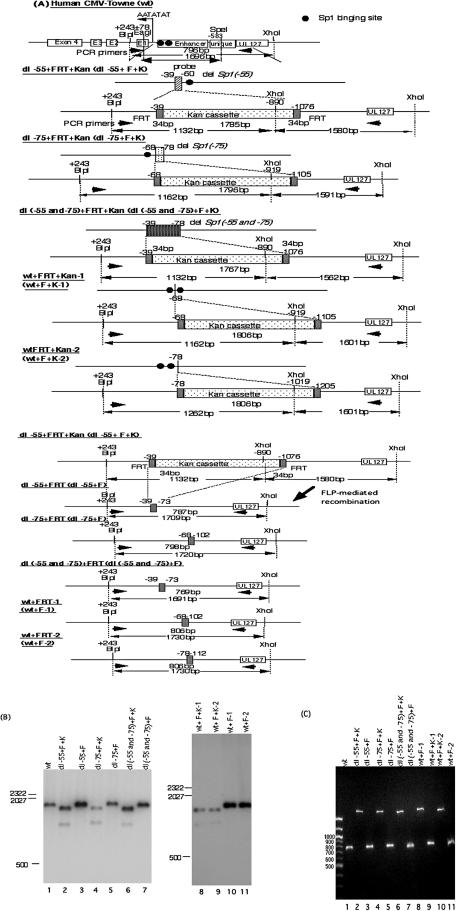
FIG. 4.
Structural analyses of recombinant HCMV BAC DNAs. (A) Schematic illustrations of parental and recombinant BAC DNAs with and without the Kanr gene. All recombinant BAC DNAs were constructed using a PCR-based rapid recombination system, and the Kanr gene was excised as described in Materials and Methods. The viral DNA fragment sizes of all recombinant viruses digested with restriction endonucleases BlpI and XhoI are indicated. Southern blot hybridization was performed with the 32P-labeled probe designated in the diagram for the human CMV Towne strain (wt). PCR was performed using primers designated in the diagram. (B) Southern blot analysis of the parental (wt) and dl−55+F, dl−75+F, dl(−55 and −75)+F, wt+F-1, and wt+F-2 recombinant BAC DNAs, with and without Kanr. Viral DNAs were digested with restriction endonucleases BlpI and XhoI, fractionated by electrophoresis in 1.0% agarose gels, and subjected to hybridization with the 32P-labeled probe. Standard molecular size markers are indicated in base pairs. BAC DNAs are identified at the tops of the blots. (C) PCR analysis of the parental and recombinant BAC DNAs. The products were amplified using the primer pair shown in panel A and fractionated by electrophoresis in 1.0% agarose and stained with ethidium bromide. BAC DNAs are identified at the top of the blot.
Northern blot analysis.
Cytoplasmic RNAs from mock-infected or HCMV-infected HFF cells were purified as described previously (9, 24), and 20-μg aliquots were subjected to electrophoresis in a 1% agarose gel containing 2.2 M formaldehyde and transferred to maximum strength Hybond N+ (Amersham). Northern blot analysis was performed as described previously (51). IE1 DNA was amplified by PCR using the primer pair ex4F (5′-AAGCGGGAGATGTGGATGGC-3′) and ex4R (5′-GGGATAGTCGCGGGTACAGG-3′) and cloned into a TA cloning vector (Invitrogen). Amplified IE1 DNA probe was generated by labeling with [32P]dCTP as described above.
Real-time PCR and reverse transcriptase PCR analysis.
For detection of low levels of MIE RNA, whole-cell RNA was purified at the times indicated using TRI reagent (Invitrogen) according to the manufacturer's instructions. Transcriptor reverse transcriptase (Roche Applied Science, Penzberg, Germany) was used according to the manufacturer's directions to generate first-strand cDNA from 2 μg of RNA and 250 ng of oligo(dT) primer (Roche) in a final volume of 20 μl. Samples were heat inactivated at 70°C for 15 min. For detection of viral DNA, cells in 60-mm plates were harvested at 4 h p.i. in triplicate with PCR lysis buffer containing 50 μg/ml proteinase K as described above. Amplifications were performed in a final volume of 25 μl containing PLATINUM Quantitative PCR SUPERMIX-UDG cocktail (Invitrogen). Each reaction mixture contained 2 μg of the first-strand cDNA or DNA, 5 mM MgCl2, and a 500 nM concentration of each MIE primer or gB primer, 250 nM MIE probe, or gB probe. MIE primers and MIE reporter probe were designed as described previously (49). HCMV gB primers were designed as 5′-GGCGAGGACAACGAAATCC-3′ and 5′-TGAGGCTGGGAAGCTGACAT-3′. The gB reporter probe was designed as 5′-FAM-TTGGGCAACCACCGCACTGAGG-tetramethyl rhodamine (TAMRA)-3′ (PE Applied Biosystems, Branchburg, NJ) as described previously (29). Thermal cycling conditions were an initial 50°C for 2 min and 95°C for 2 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Quantitation of relative MIE RNA or gB DNA was accomplished according to a standard curve analysis as described previously (49).
Viral DNA replication assay.
After infection with an equal viral DNA input, cells were collected at the times indicated. Cells in 35-mm plates in triplicate were suspended in lysis buffer (50 mM Tris-HCl, pH 8.0, 10 mM EDTA, 1% SDS, and 20 μg/ml RNaseA) containing 50 μg/ml proteinase K. After incubation at 55°C for 10 min and 95 °C for 10 min, DNA was extracted with phenol and chloroform and precipitated with ethanol. Lambda DNA (2 μg) was added to each sample after cell lysis, but before proteolysis and phenol-chloroform extraction, to control for sample-to-sample variation in processing, endonuclease digestion, and loading. Viral genomes were digested with endonuclease HindIII, fractionated in a 0.6% agarose gel and subjected to Southern blot analysis as described previously (29). The 1.6-kbp BamHI-HindIII fragment of plasmid p1.6 (T probe) was used to probe human CMV genomic termini containing terminal repeat long or inverted repeat long as described previously (50).
RESULTS
Sp1 and Sp3 transcription factors bind to the GC boxes in the proximal enhancer.
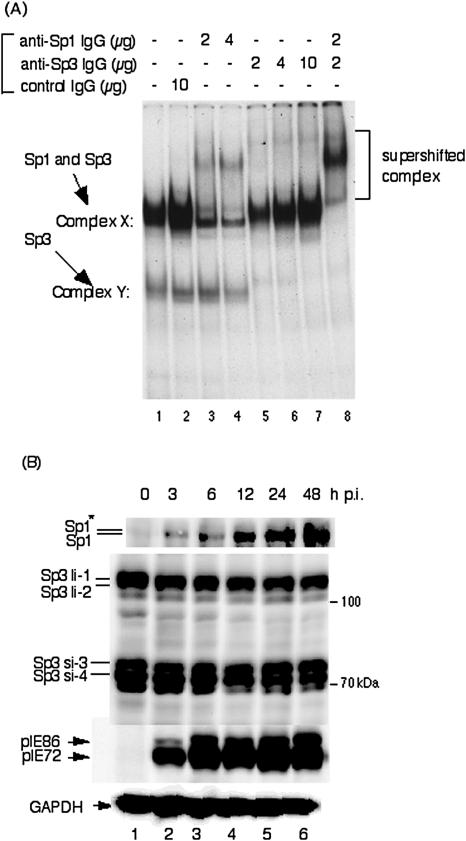
We previously described the minimal sequence required upstream of the HCMV promoter for viral replication (30). While enhancerless HCMV (−39+F) with a TATA box did not replicate in cell culture, the recombinant virus (−67+F) with the promoter and a GC box located between positions −57 and −52 relative to the transcription start site (+1) replicated. The upstream region between positions −78 and −70 also contains a GC box. The region between −116 and −67 enhanced the level of MIE transcription from the MIE promoter. To determine whether Sp1(−55) and Sp1(−75) bind either Sp1 or Sp3, an EMSA was performed. Figure 1A (lane 1) shows the results of an EMSA using the Sp1(−75) probe and the detection of complex X and complex Y. Both complexes were determined to be specific for the probe by using excess amounts of nonradioactive Sp1(−75) competitor (data not shown). Rabbit polyclonal IgG specific for Sp1 caused most of complex X to supershift but had little to no effect on complex Y. It should be noted that a portion of the complex X remained. In contrast, control IgG had no effect (Fig. 1A, lanes 3 and 4). Antibodies against Sp3 caused all of complex Y to supershift (Fig. 1A, lanes 5 to 7). A mixture of the antibodies against Sp1 and Sp3 caused all of complex X and complex Y to supershift (Fig. 1A, lane 8). The Sp1(−55) probe gave the same type of EMSA supershift results (data not shown). These data indicate that either Sp1 or Sp3 transcription factors can bind to the GC boxes at approximately positions −75 and −55 in the HCMV MIE proximal enhancer.
FIG. 1.
Sp1 and Sp3 transcription factors bind to GC boxes located in the proximal enhancer of HCMV MIE genes. (A) Supershift assay of DNA-protein complexes with anti-Sp1 and/or anti-Sp3 antibodies plus nuclear extracts using the 32P-labeled Sp1(−75) as a probe. Lane 1, probe plus nuclear extract; lane 2, probe plus nuclear extract with 10 μg of rabbit control IgG; lanes 3 and 4, probe plus nuclear extract with 2 and 4 μg of anti-Sp1 antibody, respectively; lanes 5, 6, and 7, probe plus nuclear extract with 2, 4, and 10 μg of anti-Sp3 antibody, respectively; lane 8, probe plus nuclear extract with 2 μg of anti-Sp1 and anti-Sp3 antibodies. Similar results were obtained using 32P-labeled Sp1(−55) as a probe.(B) Expression levels of Sp1 and Sp3 transcription factors after infection with HCMV. HFF cells were infected with HCMV at an MOI of 3 and harvested at the indicated times. Protein samples were prepared and 20 μg of each protein was applied for Western blot analyses. Proteins were separated by using 10% SDS-polyacrylamide gels. Detection of Sp1, Sp3, and immediate-early pIE72 (UL123)/pIE86 (UL122) was performed with polyclonal antibodies sc-59 and sc-644 and monoclonal antibody NEA-9221, respectively. Anti-pGAPDH antibody was used to confirm equal protein loading. For Sp1 the asterisk indicates the phosphorylated form. Abbreviations: Sp3li-1 and -2, long isoforms of Sp3; Sp3si-3 and -4, small isoforms of Sp3. The time p.i. is given at the top of each lane.
We also determined the effects of HCMV infection on the expression levels of Sp1 and Sp3. HFF cells were infected with wt virus at an MOI of 3 and harvested at 3, 6, 12, 24, and 48 h p.i. Equal amounts of protein were applied for SDS-polyacrylamide gel electrophoresis and Western blotting for analysis of Sp1 and Sp3 detection. Expression levels of the Sp3 isoforms, which are derived from alternative translational start sites (58), were constant throughout infection (Fig. 1B). In contrast, the levels of Sp1 increased with time after infection (Fig. 1B). These results agree with Yurochko et al. (72), who reported that HCMV infection up-regulates Sp1 levels in human fibroblasts. It is possible that Sp3 could bind to the proximal enhancer immediately after infection and that Sp1 binding could increase with time after infection.
Effect of Sp1(−55) and Sp1(−75) on MIE transcription.
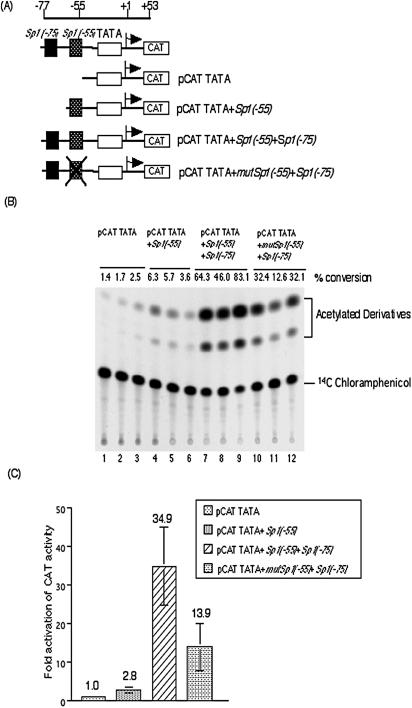
The effects of Sp1(−55) and Sp1(−75) on transcription from the MIE promoter in transient transfection assays was determined using the CAT gene constructs shown in Fig. 2A. CAT activity was determined relative to pCAT TATA, which contains only the MIE promoter TATA box. There was a low level of activity with pCAT TATA. There was a 2.8-fold increase in CAT activity for pCAT TATA+Sp1(−55) (Fig. 2B and C). pCAT TATA+Sp1(−55)+Sp1(−75) had a 34.9-fold increase in CAT activity (Fig. 2B and C). pCAT TATA+mutSp1(−55)+Sp1(−70), containing a mutant Sp1 site at position −55, had a 13.9-fold increase in CAT activity (Fig. 2B and C). The two Sp1 and Sp3 binding sites located in the MIE proximal enhancer of the HCMV enhanced transcription in transient transfection assay.
FIG. 2.
Sp1/Sp3 binding sites in the proximal enhancer have positive and synergistic effects on MIE promoter activity. (A) Schematic representation of the structures of the CAT gene constructs. (B) HFF cells were transfected with each plasmid and harvested at 72 h. CAT assays were performed as described in Materials and Methods. Experiments were performed in triplicate. Values represent percent conversion from unacetylated chloramphenicol to the acetylated form. (C) The increase in activation (n-fold) of CAT activity relative to that for pCAT TATA was determined and plotted on the graph. Data represent averages from three independent experiments.
Binding affinity at the Sp1/Sp3 binding sites.
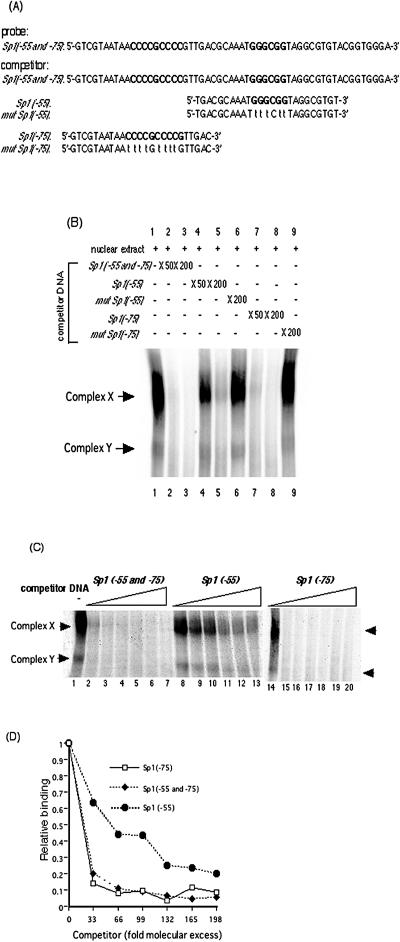
To determine the relative binding affinity of Sp1 or Sp3 for each of the Sp1/Sp3 binding sites, EMSAs were performed with a variety of competitor DNAs. Figure 3A shows the DNA sequences of the 32P-labeled probe and competitors with the Sp1/Sp3 binding sites and their mutations. Complex X and complex Y were formed with probes containing binding sites at both positions −55 and −75. The gel shift pattern appears more of a smear than is shown in Fig. 1. The Sp1(−55 and −75) probe contains two GC boxes while the Sp1(−55) or Sp1(−75) probe possesses only one. Therefore, the formed structures of the Sp1(−55 and −75) probe with Sp1 and Sp3 transcription factors are complex, and in native gel they appear to show diversity. Both complexes were reduced with excess amounts of nonradioactive competitors containing the −55 plus −75 sites (Fig. 3B, lanes 2 and 3). Competitors with either Sp1 or Sp3 binding site mutated were unable to compete for DNA complexes X and Y even at a 200-fold molar excess (Fig. 3B, lanes 6 and 9). To estimate the affinity of the transcription factors to each Sp1 or Sp3 binding site in the sequence containing both of the binding sites, nuclear extracts and probes were incubated with the indicated excess amounts (n-fold) of nonradioactive competitors containing each Sp1 or Sp3 binding site; then the reduced ratios of the detected complexes were compared between the Sp1(−55)and Sp1(−75) competitors. As shown in Fig. 3B (compare lanes 4 and 5 with lanes 7 and 8), competition for in vitro DNA binding with Sp1(−75) was stronger than with Sp1(−55).
FIG. 3.
Binding activity of Sp1/Sp3 transcription factors to Sp1(−75) and to Sp1(−55). (A) DNA sequences of 32P-labeled probe containing both Sp1(−55) and Sp1(−75) and competitor dsDNAs containing Sp1(−55 and −75), Sp1(−55), mutated Sp1(−55), Sp1(−75), or mutated Sp1(−75). The probe and the competitor dsDNAs were generated as described in Materials and Methods. (B) Autoradiogram of competitive EMSA. Competitive EMSAs were performed using 32P-labeled Sp1(−55 and −75) containing both GC boxes as a probe and the indicated excess of each competitor dsDNA as described in Materials and Methods. Lanes contain the following: probe plus nuclear extract (lane 1), probe plus nuclear extract in the presence of a 50- or 200-fold molar excess of Sp1(−55 and −75) DNA (lanes 2 and 3, respectively), probe plus nuclear extract in the presence of a 50- or 200-fold molar excess of nonradioactive Sp1(−55) DNA (lanes 4 and 5, respectively), probe plus nuclear extract in the presence of a 200-fold molar excess of nonradioactive mutated Sp1(−55) DNA (lane 6), probe plus nuclear extract in the presence of a 50- or 200-fold molar excess of nonradioactive Sp1(−75) DNA (lanes 7 and 8, respectively), probe plus nuclear extract in the presence of a 200-fold molar excess of nonradioactive mutated Sp1(−75) DNA (lane 9). (C) Binding affinity of the transcription factors for Sp1(−55) and Sp1(−75) examined quantitatively by competitive EMSA. The probe for Sp1(−55 and −75) was incubated with nuclear extracts in the absence or presence of increasing concentrations (33-, 66-, 99-, 132-, 165-, and 198-fold molar excesses) of Sp1(−55 and −75), Sp1(−55), or Sp1(−75) nonradioactive competitor DNA. Lanes contain the following: probe in the absence of competitor DNA (lanes 1 and 14), 33-fold molar excess of competitor DNA (lanes 2, 8, and 15), 66-fold molar excess of competitor DNA (lanes 3, 9, and 16), 99-fold molar excess of competitor DNA (lanes 4, 10, and 17), 132-fold molar excess of competitor DNA (lanes 5, 11, and 18), 165-fold molar excess of competitor DNA (lanes 6, 12, and 19),and 198-fold molar excess of competitor DNA (lanes 7, 13, and 20). (D) Quantification of complex X and Y formation shown in panel C. Intensities were assessed with a BAS2500 (Fuji) image analyzer as described in Materials and Methods. The results are expressed as ratios of binding in the absence of competitor DNA.
To confirm the different binding affinities of Sp1 and Sp3 for the −55 and the −75 sites, quantitative competition assays were performed. As shown Fig. 3C and D, a 33-fold molecular excess of Sp1(−75) nonradioactive competitor was sufficient to achieve a 70% reduction for complex X. In contrast, a 132-fold molecular excess of nonradioactive competitor was required for Sp1(−55). The binding of nuclear factors in vitro to Sp1(−75) is approximately fourfold stronger than to Sp1(−55).
Isolation of recombinant HCMVs with mutations in one or both of the GC boxes.
While HCMV enhancers have multiple transcription factor binding sites, it is not known whether one type of site functions more efficiently in HFF cells than another. To determine the roles of the two Sp1 and Sp3 binding sites within the MIE enhancer, we constructed recombinant BAC DNAs with each or both of the proximal Sp1 and Sp3 binding sites mutated. Since a drug resistance gene is necessary to select the recombinant BAC DNA from the parental BAC DNA, we designed the recombinant BAC DNAs to contain the kanamycin resistance gene (Kanr). After Kanr was excised by Flp-mediated recombination, only 34 bp of FRT was left in the MIE enhancer. To avoid the possibility that the FRT site itself has some effect on MIE transcription and viral replication, we also constructed two recombinant viruses with the FRT site as a control between the two Sp1/Sp3 binding sites and just upstream of the −75 site. All recombinant BAC DNAs were digested with the restriction endonucleases BlpI and XhoI. Viral DNA fragments were fractionated by electrophoresis in 1% agarose gels and immobilized for Southern blot hybridization as described in Materials and Methods. Since Kanr and the region of UL127 each have an XhoI site, recombinant BAC DNAs containing Kanr were detected as two DNA fragments by Southern blotting (Fig. 4B) . It was difficult to distinguish the size of the DNA fragments digested by BlpI and XhoI from the parental BAC DNA after excision of Kanr. Therefore, PCR analysis with the primer pairs of UL127R and HCMVF was performed for sequencing of the PCR products. As shown in Fig. 4C, the recombinant viruses had the appropriate sizes of DNA fragments predicted from Fig. 4A. Sequencing of the amplified DNA fragments confirmed the correct mutation, recombination, and excision (data not shown). To isolate the recombinant viruses, HFF cells were transfected with the recombinant HCMV BAC DNAs as described in Materials and Methods.
Effect of mutation of the Sp1/Sp3 binding sites on MIE transcription in infected cells.
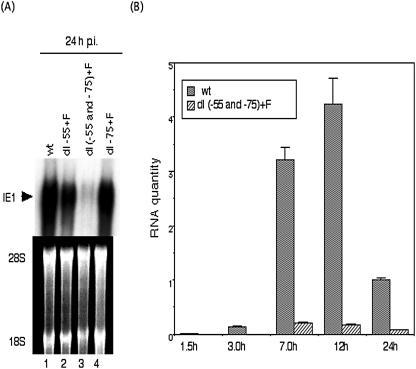
To determine the role of the two Sp1/Sp3 binding sites on the MIE promoter in the context of HCMV infection, we compared the levels of IE1 gene transcripts by Northern blot analysis. The amount of viral DNA input for the various recombinant viruses was determined by quantitative real-time PCR assay at 4 h p.i. as described in Materials and Methods. After infection with an equal viral DNA input, equivalent to an MOI of approximately 0.5 for wild type, RNAs were harvested at 24 h p.i. and subjected to agarose gel electrophoresis. Ethidium bromide staining of 28S and 18S rRNA confirmed that equal amounts of RNA were loaded in each lane (Fig. 5A). Significant differences in the levels of steady-state IE1 RNA transcripts between wt and the recombinant viruses with mutations in either Sp1(−55) or Sp1(−75) were not detected. In contrast, the IE1 RNA level for the recombinant virus with mutations in both the Sp1 and Sp3 binding sites was significantly lower (Fig. 5A, lane 3).
FIG. 5.
Viral MIE gene transcription with the wt and recombinant viruses. (A) Steady-state IE1 mRNA levels at 24 h p.i. were analyzed with an IE1 probe by Northern blotting as described in Materials and Methods. 28S and 18S ribosomal RNAs served as controls for equal amounts of RNA loading. (B) Kinetics of MIE gene transcription for the wt and dl(−55 and −75)+F recombinant viruses, assayed by real-time PCR. HFF cells were infected with the wt or dl(−55 and −75)+F recombinant virus at an MOI of 3. Total RNAs were purified in parallel at 1.5, 3.0, 7.0, 12, and 24 h p.i., and levels of IE1 and IE2 transcripts were analyzed by real-time PCR as described in Materials and Methods. Values were calculated relative to the level of the wt transcripts at 24 h.p.i. Data are averages of three independent experiments.
The steady-state IE1 and IE2 RNA levels were quantitated by real-time reverse transcriptase PCR assay at various times after infection with wt or recombinant virus with both Sp1 and Sp3 mutated at an MOI of 3. The wt IE RNA levels reached a maximum at 12 h p.i. and then decreased, coinciding well with a previous report (51). Viral IE RNA was more than 10-fold lower with recombinant virus RdlSp1(−55 and −75)+F from 3 to 24 h p.i. These data indicate that at high MOI either the −75 or the −55 site will function for efficient MIE transcription but that without at least one GC box site, MIE transcription is very inefficient.
Effects of mutation of the Sp1 and Sp3 binding sites on viral DNA replication at a high or low MOI.
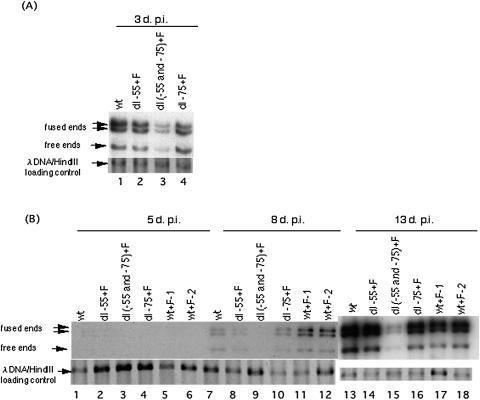
To determine the effects of the two Sp1 and Sp3 binding sites on viral DNA replication, HFF cells were infected with wt or the recombinant virus at MOIs of 3 or 0.01, with verification of similar viral input as judged by real-time PCR analysis using gB primers as described in Materials and Methods. DNAs from equal numbers of infected cells were purified, and viral DNA was quantified by Southern blot hybridization with HCMV genomic termini as a probe. Lambda DNA served as a loading control. At high MOI (MOI of 3) DNA replication of the recombinant viruses with deletion of either of the Sp1 or Sp3 binding sites was almost similar to that of wt at 3 days p.i. In contrast, the replication of Rdl(−55 and −75)+F was reduced threefold (Fig. 6A). At low MOI, DNA replication with Rdl-55+F or Rdl-75+F did not differ significantly from wt. In contrast, DNA replication of Rdl(−55 and −75)+F was approximately 46- and 12-fold less at 8 and 13 days p.i., respectively. These data indicate that at least one Sp1 or Sp3 site is necessary for viral DNA replication in HFF cells.
FIG. 6.
Effect of deletion of the two Sp1/Sp3 binding sites on viral DNA replication at high and low MOIs. (A) HFF cells were infected with wt or dl-55+F, dl-75+F, or dl(−55 and −75)+F recombinant viruses at an MOI of 3 and harvested 3 days p.i. DNAs purified from cells were digested with restriction endonuclease HindIII and subjected to Southern blot hybridization with the 32P-labeled T probe as described in Materials and Methods. Arrows designate the viral DNA fused ends 17.2- and 13.0-kbp, free ends 9.7-kbp, and internal lambda DNA control. (B) HFF cells were infected with wt or wt+F-1, wt+F-2, dl-55+F, dl-75+F or dl(−55 and −75)+F recombinant viruses at an MOI of 0.01 and harvested 5, 8, and 13 days p.i. DNAs purified from cells were digested with HindIII and subjected to Southern blot hybridization with the 32P-labeled T probe.
Effects of mutation of the Sp1 and Sp3 binding sites on viral plaque size.
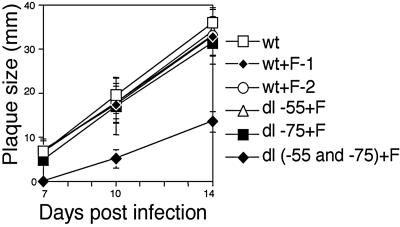
To determine the effects of the Sp1 and Sp3 binding sites on infectious virus replication and plaque size, we measured plaque sizes of wt and recombinant viruses. Viral plaque size reflects multiple rounds of viral replication (29). All plaques were generated from inocula infected with wt or recombinant virus at an MOI of 0.01, and agar was overlaid 3 days p.i. Plaque sizes were determined at 7, 10, and 14 days p.i. With Sp1(−55) or Sp1(−75) mutated, plaque size was not reduced. With both Sp1 and Sp3 binding sites mutated, plaque size was significantly smaller at 10 and 14 days p.i. (Fig. 7).
FIG. 7.
Plaque formation after infection with recombinant virus having both Sp1/Sp3 binding sites deleted. All plaques were generated from inocula infected with wt or wt+F-1, wt+F-2, dl-55+F, dl-75+F or dl(−55 and −75)+F recombinant viruses at an MOI of 0.01 in HFF cells. Each plaque size was determined as a mean of minimum and maximum lengths at the indicated days after infection. The results are averages for 10 plaques.
DISCUSSION
HCMV MIE gene expression is essential for viral replication. Enhancerless HCMV even with the TATA box containing promoter element does not replicate in cell culture (30). The Sp1/Sp3 binding sites near the TATA box of the MIE promoter have a critical role in efficient MIE transcription and in viral replication.
The protein level of transcription factor Sp3 was constant throughout infection, while the level of Sp1 increased as the infection proceeded. It is likely that the increased binding affinity of Sp1 promoted the transcription from the MIE promoter after infection. Yurochko et al. has reported that there was an increase in the binding affinity of Sp1 to the GC box immediately after HCMV infection, and the attachment of HCMV glycoprotein gB and gH are responsible for the induction (70, 71). Other DNA viruses, like adeno-associated virus and human papillomavirus (2, 8, 38, 55), also utilize Sp1-like transcription factors to obtain efficient early viral gene expression in specific cell types.
The role of NF-κB sites in the HCMV enhancer for MIE gene expression has been questioned (4). The Sp1/Sp3 binding sites in the proximal enhancer of HCMV cannot be fully compensated by other transcription factor binding sites. At least one Sp1 or Sp3 binding site is required for the efficient transcription from the MIE promoter in HFF cells. The reasons why either site alone can function like wild type and, consequently, the sites are redundant are still unclear. The virion-associated factors such as glycoprotein gB and gH (70) and tegment protein pp71 (3, 6, 44) would influence the enhancer containing MIE promoter in the recombinant virus with the one Sp1 or Sp3 binding site mutated. Whether in the other cell types such as epithelial cells, endothelial cells, and monocyte-derived macrophages both Sp1/Sp3 binding sites are necessary remained to be determined. In contrast, mutation of the four NF-κB sites in the HCMV enhancer failed to alter viral replication at high or low MOIs (4). In addition, mutation of all five CREB/ATF sites in the HCMV enhancer also failed to alter MIE gene expression (32). It is likely that other transcription factors in the complicated MIE enhancer can compensate for CREB/ATF or NF-κB in recombinant virus-infected HFF cells.
Sp1 is a ubiquitous transcription factor that regulates the constitutive expression of many cellular genes. While recognition of the TATA box by the TATA-binding protein and TATA-binding protein-associated factors (TAFs) is essential for minimal promoter activity, Sp1 may interact with TAF4 (57) for synergistic effects on transcription from the HCMV MIE promoter (11). Sp1 is also involved in activation of an initiator sequence (Inr) at the transcription start site (16). The HCMV MIE promoter has a Inr-like sequence as described previously (46). TAFs also recognize the Inr and promote direct interaction with RNA polymerase II (67, 68). Sp1 stimulates activity from a promoter with a TATA box and an Inr element more strongly than from a promoter with just a TATA box (16). It is possible that Sp1 or Sp3 promotes the initial transcription from the MIE promoter.
Sp1 also activates promoters through interaction with transcription factors. For example, Sp1 activates the promoter of the rat Na,K-ATPase alpha 1 subunit gene (35) through its interaction with CREB and CBP (54, 66). In the HCMV-infected cell, Sp1 may act as a critical bridge between the HCMV MIE TATA box and the transcription factors binding to the proximal and distal enhancers.
Histone deacetylases are reported to repress the HCMV MIE promoter in nonpermissive cells, and inhibition of histone deacetylases induces viral permissiveness (48, 53). Sp1 affects histone acetylation within a proximal enhancer. For example, an Sp1 binding site upstream of the monocyte chemoattractant protein 1 gene is required for histone acetylation (5). The effect of Sp1 on the acetylation of histones associated with the HCMV MIE enhancer may be essential for transcription from the MIE promoter during the switch from latent to lytic infection.
Murine and simian CMVs also have Sp1/Sp3 binding sites in the proximal enhancer near the TATA box (10, 13, 31). Since betaherpesviruses have cell type and species specificities for productive viral replication, an Sp1/Sp3 binding site(s) in the proximal enhancer of CMVs may promote permissive infection of HFF cells.
Acknowledgments
We thank P. E. Lashmit and Y. Nishikawa for technical assistance, G. Suske and J. Toguchida for helpful suggestions about Sp3, and S. Wang for critical reading of the manuscript.
This work was supported by Grants-in-aid for Scientific Research on Priority Areas from the Ministry of Education, Science, Sports, Culture and Technology of Japan (14021138 and 12470073 to T.T.), Research on Health Sciences focusing on Drug Innovation (SH54412 to H.I.), and grant AI-13562 from the National Institutes of Health (to M.F.S.).
REFERENCES
- 1.Aoyama, T., T. Okamoto, S. Nagayama, K. Nishijo, T. Ishibe, K. Yasura, T. Nakayama, T. Nakamura, and J. Toguchida. 2004. Methylation in the core-promoter region of the chondromodulin-I gene determines the cell-specific expression by regulating the binding of transcriptional activator Sp3. J. Biol. Chem. 279:28789-28797. [DOI] [PubMed] [Google Scholar]
- 2.Apt, D., R. M. Watts, G. Suske, and H. U. Bernard. 1996. High Sp1/Sp3 ratios in epithelial cells during epithelial differentiation and cellular transformation correlate with the activation of the HPV-16 promoter. Virology 224:281-291. [DOI] [PubMed] [Google Scholar]
- 3.Baldick, C. J., A. Marchini, C. E. Patterson, and T. Shenk. 1997. Human cytomegalovirus tegument protein pp71 (ppUL82) enhances the infectivity of viral DNA and accelerates the infectious cycle. J. Virol. 71:4400-4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benedict, C. A., A. Angulo, G. Patterson, S. Ha, H. Huang, M. Messerle, C. F. Ware, and P. Ghazal. 2004. Neutrality of the canonical NF-κB-dependent pathway for human and murine cytomegalovirus transcription and replication in vitro. J. Virol. 78:741-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boekhoudt, G. H., Z. Guo, G. W. Beresford, and J. M. Boss. 2003. Communication between NF-κB and Sp1 controls histone acetylation within the proximal promoter of the monocyte chemoattractant protein 1 gene. J. Immunol. 170:4139-4147. [DOI] [PubMed] [Google Scholar]
- 6.Bresnahan, W. A., and T. E. Shenk. 2000. UL82 virion protein activates expression of immediate early viral genes in human cytomegalovirus-infected cells. Proc. Natl. Acad. Sci. USA 97:14506-14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Britt, W. J., and C. A. Alford. 1996. Cytomegaloviruses, p. 2493-2523. In D. M. Knipe, B. Roizman, P. M. Howley, T. P. Monath, S. E. Straus, R. M. Chanock, J. L. Melnick, and B. N. Fields (ed.), Fields virology, 3rd ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 8.Butz, K., and F. Hoppe-Seyler. 1993. Transcriptional control of human papillomavirus (HPV) oncogene expression: composition of the HPV type 18 upstream regulatory region. J. Virol. 67:6476-6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang, C.-P., C. L. Malone, and M. F. Stinski. 1989. A human cytomegalovirus early gene has three inducible promoters that are regulated differentially at various times after infection. J. Virol. 63:281-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang, Y. N., S. Crawford, J. Stall, D. R. Rawlins, K. T. Jeang, and G. S. Hayward. 1990. The palindromic series I repeats in the simian cytomegalovirus major immediate-early promoter behave as both strong basal enhancers and cyclic-AMP response elements. J. Virol. 64:264-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, J. L., L. D. Attardi, C. P. Verrijzer, K. Yokomori, and R. Tjian. 1994. Assembly of recombinant TFIID reveals differential coactivator requirements for distinct transcriptional activators. Cell 79:93-105. [DOI] [PubMed] [Google Scholar]
- 12.Cherrington, J. M., E. L. Khoury, and E. S. Mocarski. 1991. Human cytomegalovirus ie2 negatively regulates α gene expression via a short target sequence near the transcription start site. J. Virol. 65:887-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorsch-Hasler, K., G. M. Keil, F. Weber, J. M. Schaffner, and U. H. Koszinowski. 1985. A long and complex enhancer activates transcription of the gene coding for the highly abundant immediate early mRNA in murine cytomegalovirus. Proc. Natl. Acad. Sci. USA 82:8325-8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunn, W., C. Chou, H. Li, R. Hai, D. Patterson, V. Stolc, H. Zhu, and F. Liu. 2003. Functional profiling of a human cytomegalovirus genome. Proc. Natl. Acad. Sci. USA 100:14223-14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellis, H. M., D. Yu, T. DiTizio, and D. L. Court. 2001. High efficiency mutagenesis, repair, and engineering of chromosomal DNA using single-stranded oligonucleotides. Proc. Natl. Acad. Sci. USA 98:6742-6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emami, K. H., W. W. Navarre, and S. T. Smale. 1995. Core promoter specificities of the Sp1 and VP16 transcriptional activation domains. Mol. Cell. Biol. 15:5906-5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fish, K. N., W. Britt, and J. A. Nelson. 1996. A novel mechanism for persistence of human cytomegalovirus in macrophages. J. Virol. 70:1855-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fish, K. N., A. S. Depto, A. V. Moses, W. Britt, and J. A. Nelson. 1995. Growth kinetics of human cytomegalovirus are altered in monocyte-derived macrophages. J. Virol. 69:3737-3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gawn, J. M., and R. F. Greaves. 2002. Absence of IE1 p72 protein function during low-multiplicity infection by human cytomegalovirus results in a broad block to viral delayed-early gene expression. J. Virol. 76:4441-4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graham, F. L., and A. J. van der Eb. 1973. A new technique for the assay of infectivity of adenovirus 5 DNA. Virology 52:456-467. [DOI] [PubMed] [Google Scholar]
- 21.Greaves, R. F., and E. S. Mocarski. 1998. Defective growth correlates with reduced accumulation of viral DNA replication protein after low-multiplicity infection by a human cytomegalovirus ie1 mutant. J. Virol. 72:366-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hahn, G., R. Jores, and E. S. Mocarski. 1998. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proc. Natl. Acad. Sci. USA 95:3937-3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hahn, G., D. Rose, M. Wagner, S. Rhiel, and M. A. McVoy. 2003. Cloning of the genomes of human cytomegalovirus strains Toledo, TownevarRIT3, and Towne long as BACs and site-directed mutagenesis using a PCR-based technique. Virology 307:164-177. [DOI] [PubMed] [Google Scholar]
- 24.Hermiston, T. W., C. L. Malone, P. R. Witte, and M. F. Stinski. 1987. Identification and characterization of the human cytomegalovirus immediate-early region 2 gene that stimulates gene expression from an inducible promoter. J. Virol. 61:3214-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho, M. 1991. Cytomegalovirus: biology and infection. Plenum Publishing Corp., New York, N.Y.
- 26.Huang, L., and M. F. Stinski. 1995. Binding of cellular repressor protein or the IE2 protein to a cis-acting negative regulatory element upstream of a human cytomegalovirus early promoter. J. Virol. 69:7612-7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ibanez, C. E., R. Schrier, P. Ghazal, C. Wiley, and J. A. Nelson. 1991. Human cytomegalovirus productively infects primary differentiated macrophages. J. Virol. 65:6581-6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ihn, H., and M. Trojanowska. 1997. Sp3 is a transcriptional activator of the human alpha2(I) collagen gene. Nucleic Acids Res. 25:3712-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isomura, H., and M. F. Stinski. 2003. The human cytomegalovirus major immediate-early enhancer determines the efficiency of immediate-early gene transcription and viral replication in permissive cells at low multiplicity of infection. J. Virol. 77:3602-3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isomura, H., T. Tsurumi, and M. F. Stinski. 2004. Role of the proximal enhancer of the major immediate-early promoter in human cytomegalovirus replication. J. Virol. 78:12788-12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeang, K. F., D. R. Rawlins, P. J. Rosenfeld, J. H. Shero, T. J. Kelly, and G. S. Hayward. 1987. Multiple tandemly repeated binding sites for cellular nuclear factor 1 that surround the major immediate-early promoters of simian and human cytomegalovirus. J. Virol. 61:1559-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keller, M. J., D. G. Wheeler, E. Cooper, and J. L. Meier. 2003. Role of the human cytomegalovirus major immediate-early promoter's 19-base-pair-repeat cyclic AMP-response element in acutely infected cells. J. Virol. 77:6666-6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kingsley, C., and A. Winoto. 1992. Cloning of GT box-binding proteins: a novel Sp1 multigene family regulating T-cell receptor gene expression. Mol. Cell. Biol. 12:4251-4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitazawa, S., R. Kitazawa, and S. Maeda. 1999. Transcriptional regulation of rat cyclin D1 gene by CpG methylation status in promoter region. J. Biol. Chem. 274:28787-28793. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi, M., and K. Kawakami. 1997. Synergism of the ATF/CRE site and GC box in the housekeeping Na,K-ATPase alpha 1 subunit gene is essential for constitutive expression. Biochem. Biophys. Res. Commun. 241:169-174. [DOI] [PubMed] [Google Scholar]
- 36.Kondo, K., H. Kaneshima, and E. S. Mocarski. 1994. Human cytomegalovirus latent infection of granulocyte-macrophage progenitors. Proc. Natl. Acad. Sci. USA 91:11879-11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kondo, K., J. Xu, and E. S. Mocarski. 1996. Human cytomegalovirus latent gene expression in granulocyte-macrophage progenitors in culture and in seropositive individuals. Proc. Natl. Acad. Sci. USA 93:11137-11142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lackner, D. F., and N. Muzyczka. 2002. Studies of the mechanism of transactivation of the adeno-associated virus p19 promoter by Rep protein. J. Virol. 76:8225-8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lashmit, P. E., J. L. Meier, and M. F. Stinski. 2002. Mutation of a consensus Fox (HNF-3) protein binding site upstream of the human cytomegalovirus UL127 promoter results in a loss of repression of gene expression. J. Virol. 78:5113-5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lashmit, P. E., M. F. Stinski, E. A. Murphy, and G. C. Bullock. 1998. A cis repression sequence adjacent to the transcription start site of the human cytomegalovirus US3 gene is required to down regulate gene expression at early and late times after infection. J. Virol. 72:9575-9584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lathey, J. L., and S. A. Spector. 1991. Unrestricted replication of human cytomegalovirus in hydrocortisone-treated macrophages. J. Virol. 65:6371-6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang, Y., D. F. Robinson, J. Dennig, G. Suske, and W. E. Fahl. 1996. Transcriptional regulation of the SIS/PDGF-B gene in human osteosarcoma cells by the Sp family of transcription factors. J. Biol. Chem. 271:11792-11797. [DOI] [PubMed] [Google Scholar]
- 43.Liu, B., T. W. Hermiston, and M. F. Stinski. 1991. A cis-acting element in the major immediate early (IE) promoter of human cytomegalovirus is required for negative regulation by IE2. J. Virol. 65:897-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu, B., and M. F. Stinski. 1992. Human cytomegalovirus contains a tegument protein that enhances transcription from promoters with upstream ATF and AP-1 cis-acting elements. J. Virol. 66:4434-4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lundquist, C. A., J. L. Meier, and M. F. Stinski. 1999. A strong negative transcriptional regulatory region between the human cytomegalovirus UL127 gene and the major immediate-early enhancer. J. Virol. 73:9039-9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Macias, M. P., L. Huang, P. E. Lashmit, and M. F. Stinski. 1996. Cellular and viral protein binding to a cytomegalovirus promoter transcription initiation site: effects on transcription. J. Virol. 70:3628-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marchini, A., H. Liu, and H. Zhu. 2001. Human cytomegalovirus with IE-2 (UL122) deleted fails to express early lytic genes. J. Virol. 75:1870-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meier, J. L. 2001. Reactivation of the human cytomegalovirus major immediate-early regulatory region and viral replication in embryonal NTera2 cells: role of trichostatin A, retinoic acid, and deletion of the 21-base-pair repeats and modulator. J. Virol. 75:1581-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meier, J. L., M. J. Keller, and J. J. McCoy. 2002. Requirement of multiple cis-acting elements in the human cytomegalovirus major immediate-early distal enhancer for viral gene expression and replication. J. Virol. 76:313-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meier, J. L., and J. A. Pruessner. 2000. The human cytomegalovirus major immediate-early distal enhancer region is required for efficient viral replication and immediate-early gene expression. J. Virol. 74:1602-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meier, J. L., and M. F. Stinski. 1997. Effect of a modulator deletion on transcription of the human cytomegalovirus major immediate-early genes in infected undifferentiated and differentiated cells. J. Virol. 71:1246-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meier, J. L., and M. F. Stinski. 1996. Regulation of human cytomegalovirus immediate-early gene expression. Intervirology 39:331-342. [DOI] [PubMed] [Google Scholar]
- 53.Murphy, J. C., W. Fischle, E. Verdin, and J. H. Sinclair. 2002. Control of cytomegalovirus lytic gene expression by histone acetylation. EMBO J. 21:1112-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Owen, G. I., J. K. Richer, L. Tung, G. Takimoto, and K. B. Horwitz. 1998. Progesterone regulates transcription of the p21(WAF1) cyclin-dependent kinase inhibitor gene through Sp1 and CBP/p300. J. Biol. Chem. 273:10696-10701. [DOI] [PubMed] [Google Scholar]
- 55.Pereira, D. J., and N. Muzyczka. 1997. The cellular transcription factor SP1 and an unknown cellular protein are required to mediate Rep protein activation of the adeno-associated virus p19 promoter. J. Virol. 71:1747-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pizzorno, M. C., and G. S. Hayward. 1990. The IE2 gene products of human cytomegalovirus specifically down-regulate expression from the major immediate-early promoter through a target located near the cap site. J. Virol. 64:6154-6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saluja, D., M. F. Vassallo, and N. Tanese. 1998. Distinct subdomains of human TAFII130 are required for interactions with glutamine-rich transcriptional activators. Mol. Cell. Biol. 18:5734-5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sapetschnig, A., F. Koch, G. Rischitor, T. Mennenga, and G. Suske. 2004. Complexity of translationally controlled transcription factor Sp3 isoform expression. J. Biol. Chem. 279:42095-42105. [DOI] [PubMed] [Google Scholar]
- 59.Sinzger, C., A. Grefte, B. Plachter, A. S. H. Gouw, T. Hauw The, and G. Jahn. 1995. Fibroblasts, epithelial cells, endothelial cells, and smooth muscle cells are the major targets of human cytomegalovirus infection in lung and gastrointestinal tissues. J. Gen. Virol. 76:741-750. [DOI] [PubMed] [Google Scholar]
- 60.Sinzger, C., B. Plachter, A. Grefte, A. S. H. Gouw, T. H. The, and G. Jahn. 1996. Tissue macrophages are infected by human cytomegalovirus. J. Infect. Dis. 173:240-245. [DOI] [PubMed] [Google Scholar]
- 61.Stinski, M. F. 1999. Cytomegalovirus promoter for expression in mammalian cells, p. 211-233. In J. M. Ferandez and J. P. Hoeffler (ed.), Gene expression systems: using nature for the art of expression. Academic Press, San Diego, Calif.
- 62.Stinski, M. F. 1978. Sequence of protein synthesis in cells infected by human cytomegalovirus: early and late virus-induced polypeptides. J. Virol. 26:686-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Supp, D. M., D. P. Witte, W. W. Branford, E. P. Smith, and S. S. Potter. 1996. Sp4, a member of the Sp1-family of zinc finger transcription factors, is required for normal murine growth, viability, and male fertility. Dev. Biol. 176:284-299. [DOI] [PubMed] [Google Scholar]
- 64.Suske, G. 1999. The Sp-family of transcription factors. Gene 238:291-300. [DOI] [PubMed] [Google Scholar]
- 65.Taylor-Wiedeman, J., J. G. Sissons, L. K. Borysiewicz, and J. H. Sinclair. 1991. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J. Gen. Virol. 72:2059-2064. [DOI] [PubMed] [Google Scholar]
- 66.Tsai, E. Y., J. V. Falvo, A. V. Tsytsykova, A. K. Barczak, A. M. Reimold, L. H. Glimcher, M. J. Fenton, D. C. Gordon, I. F. Dunn, and A. E. Goldfeld. 2000. A lipopolysaccharide-specific enhancer complex involving Ets, Elk-1, Sp1, and CREB binding protein and p300 is recruited to the tumor necrosis factor alpha promoter in vivo. Mol. Cell. Biol. 20:6084-6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verrijzer, C. P., J. L. Chen, K. Yokomori, and R. Tjian. 1995. Binding of TAFs to core elements directs promoter selectivity by RNA polymerase II. Cell 81:1115-1125. [DOI] [PubMed] [Google Scholar]
- 68.Verrijzer, C. P., K. Yokomori, J. L. Chen, and R. Tjian. 1994. Drosophila TAFII150: similarity to yeast gene TSM-1 and specific binding to core promoter DNA. Science 264:933-941. [DOI] [PubMed] [Google Scholar]
- 69.Wathen, M. W., and M. F. Stinski. 1982. Temporal patterns of human cytomegalovirus transcription: mapping the viral RNAs synthesized at immediate early, early, and late times after infection. J. Virol. 41:462-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yurochko, A. D., E.-S. Hwang, L. Rasmussen, S. Keay, L. Pereira, and E.-S. Huang. 1997. The human cytomegalovirus UL55 (gB) and UL75 (gH) glycoprotein ligands initiate the rapid activation of Sp1 and NF-κB during infection. J. Virol. 71:5051-5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yurochko, A. D., T. F. Kowalik, S. M. Huong, and E. S. Huang. 1995. Human cytomegalovirus upregulates NF-kappa B activity by transactivating the NF-kappa B p105/p50 and p65 promoters. J. Virol. 69:5391-5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yurochko, A. D., M. W. Mayo, E. E. Poma, A. S. Baldwin, Jr., and E. S. Huang. 1997. Induction of the transcription factor Sp1 during human cytomegalovirus infection mediates upregulation of the p65 and p105/p50 NF-kappaB promoters. J. Virol. 71:4638-4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao, L., and L. S. Chang. 1997. The human POLD1 gene. Identification of an upstream activator sequence, activation by Sp1 and Sp3, and cell cycle regulation. J. Biol. Chem. 272:4869-4882. [PubMed] [Google Scholar]
- 74.Zhu, W. G., K. Srinivasan, Z. Dai, W. Duan, L. J. Druhan, H. Ding, L. Yee, M. A. Villalona-Calero, C. Plass, and G. A. Otterson. 2003. Methylation of adjacent CpG sites affects Sp1/Sp3 binding and activity in the p21(Cip1) promoter. Mol. Cell. Biol. 23:4056-4065. [DOI] [PMC free article] [PubMed] [Google Scholar]