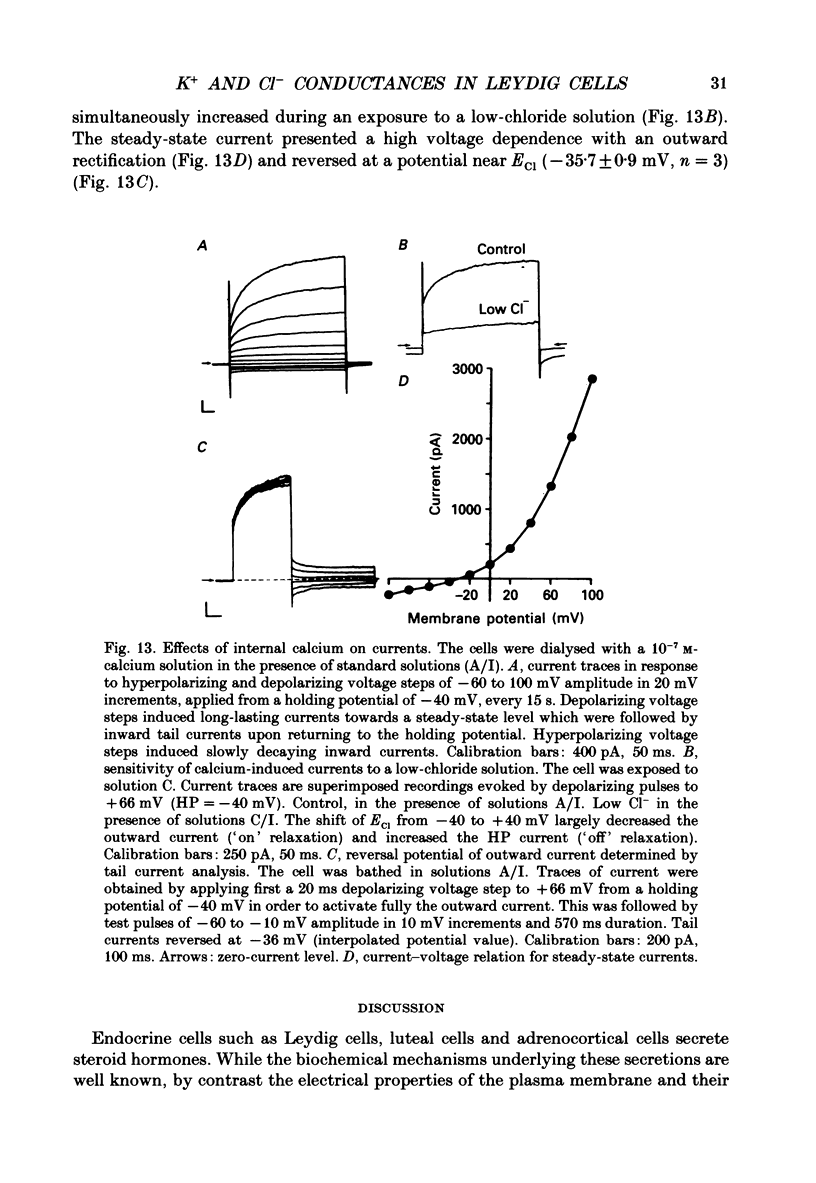
Abstract
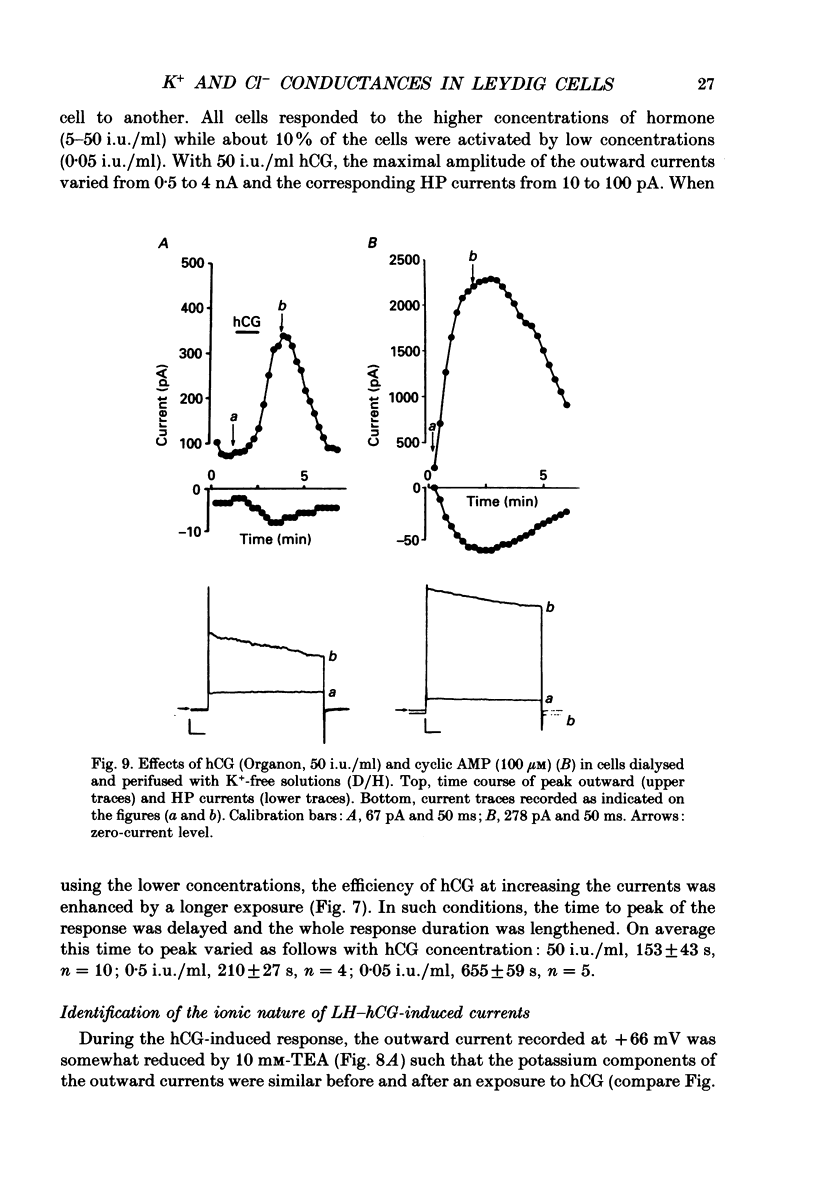
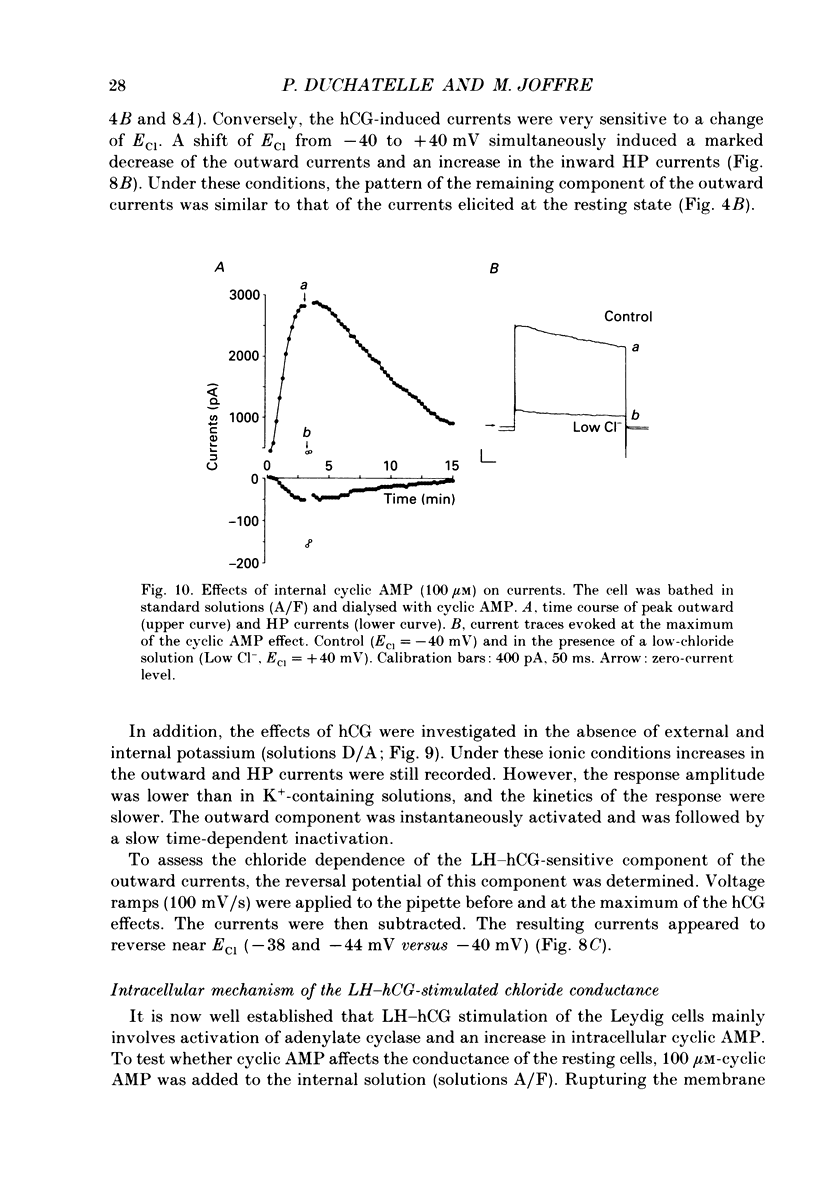
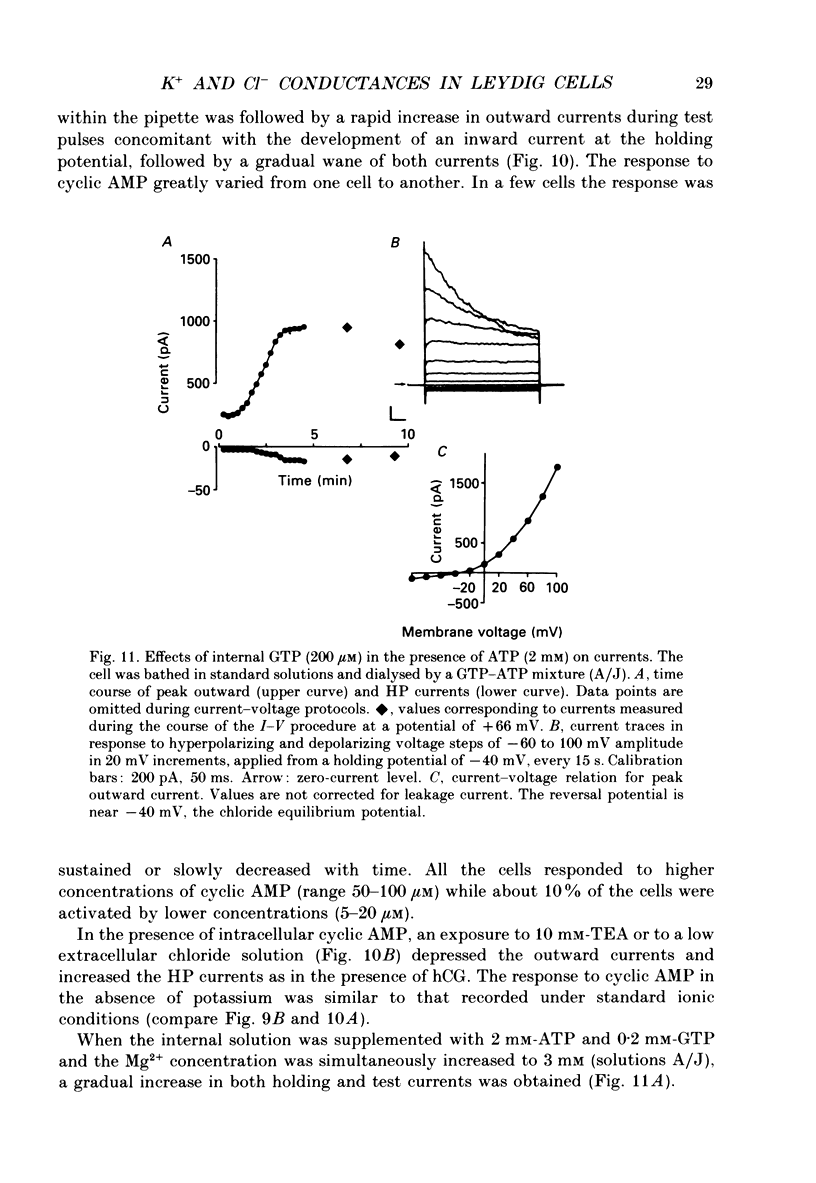
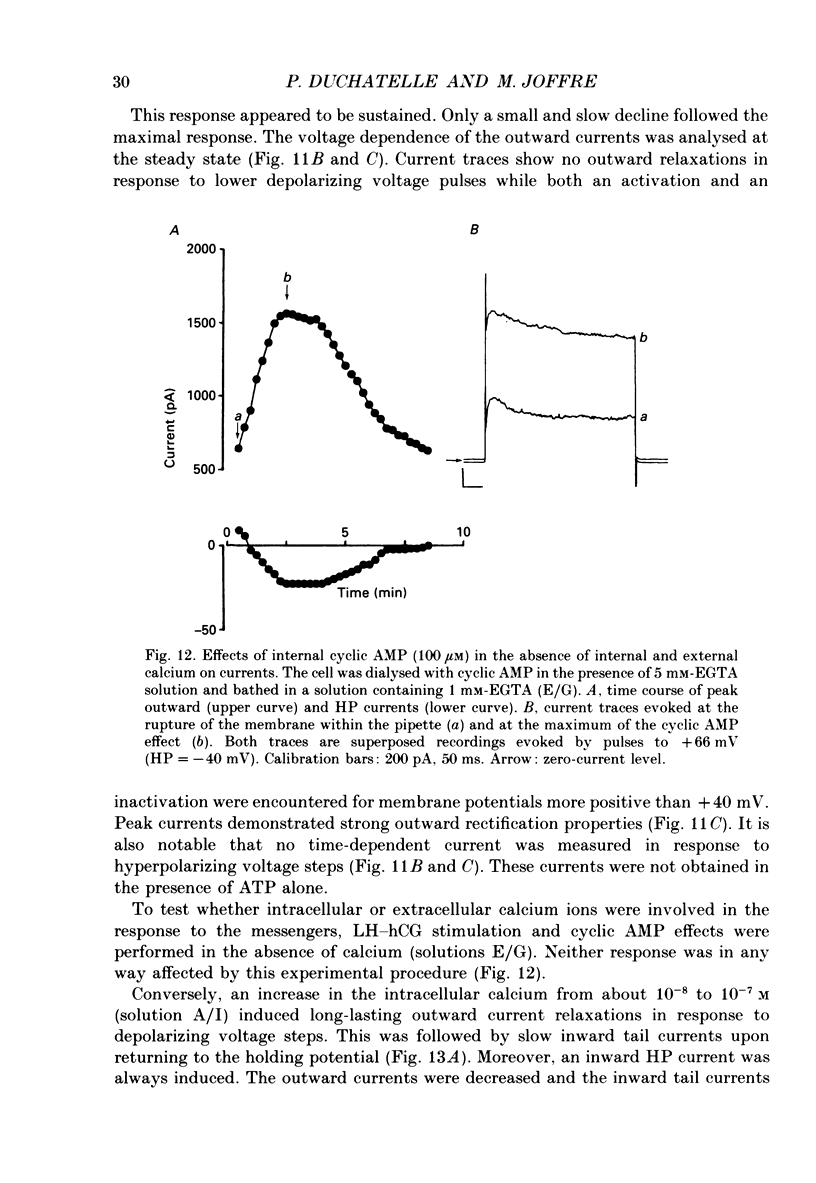
1. The effects of gonadotrophins (luteinizing hormone and human chorionic gonadotrophin) and cyclic AMP on ionic conductances were investigated using the tight-seal whole-cell recording technique in Leydig cells freshly isolated from nature rat testis by enzymatic treatment. 2. In resting cells, the predominant ionic conductance is a voltage-dependent K+ conductance resembling the delayed rectifier K+ conductance of T-lymphocytes. This conductance is characterized by: (1) a time-dependent inactivation for potentials more positive than +20 mV, (2) a reversal potential near -65 mV, (3) a sensitivity to intracellular Cs+, and (4) a sensitivity to extracellular TEA and 4-aminopyridine. 3. A Cl- conductance is also present resembling the Cl- background conductance in squid axons and heart cells. In resting cells, this conductance contributes only a small component of the total outward current obtained with depolarizing pulses. 4. Gonadotrophins (human chorionic gonadotrophin, porcine luteinizing hormone and ovine luteinizing hormone) have little effect on the K+ conductance. They transiently increase a Cl- conductance after a delay of up to 30 s. This response does not occur if the hormones are applied late in the whole-cell recording. Gonadoliberine (GnRH) does not affect the Cl- or K+ conductance. 5. Internal cyclic AMP (100 microM) mimics all these effects while internal application of a GTP-ATP mixture induces a similar response, which is, however, sustained rather than transient. 6. The Cl- conductance was studied quantitatively with a GTP-ATP internal solution. This conductance is activated by depolarizing voltage steps to test potentials of -40 mV or more. Under these conditions, the instantaneous current observed as soon as the depolarizing pulse is applied displays outward rectification and reverses near ECl. During the pulses, a strong inactivation is observed for potentials greater than +40 mV. This conductance is independent of external and internal calcium. 7. It is concluded that the gonadotrophins act through a cyclic AMP-dependent process to activate a Cl- conductance. This conductance is different to the hyperpolarization-activated Cl- conductance and the calcium-activated Cl-conductance also present in the membrane of resting cells.
Full text
PDF



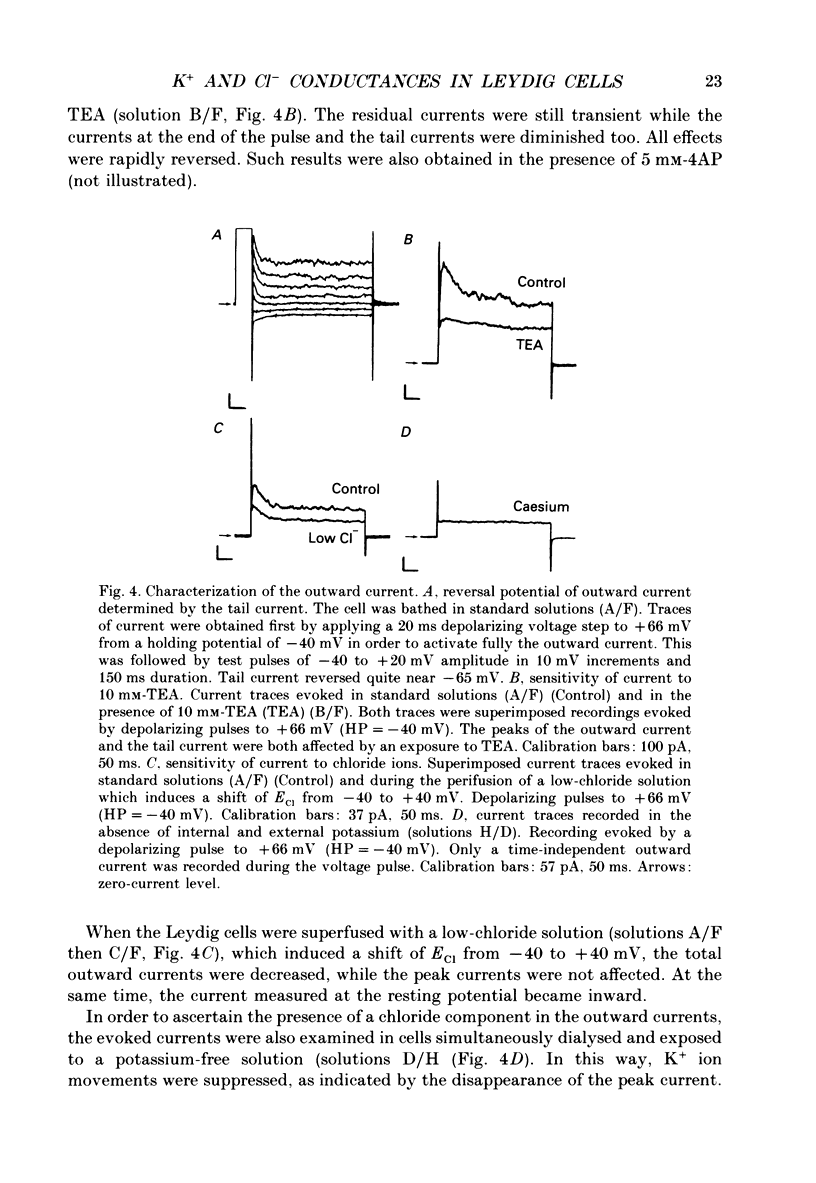
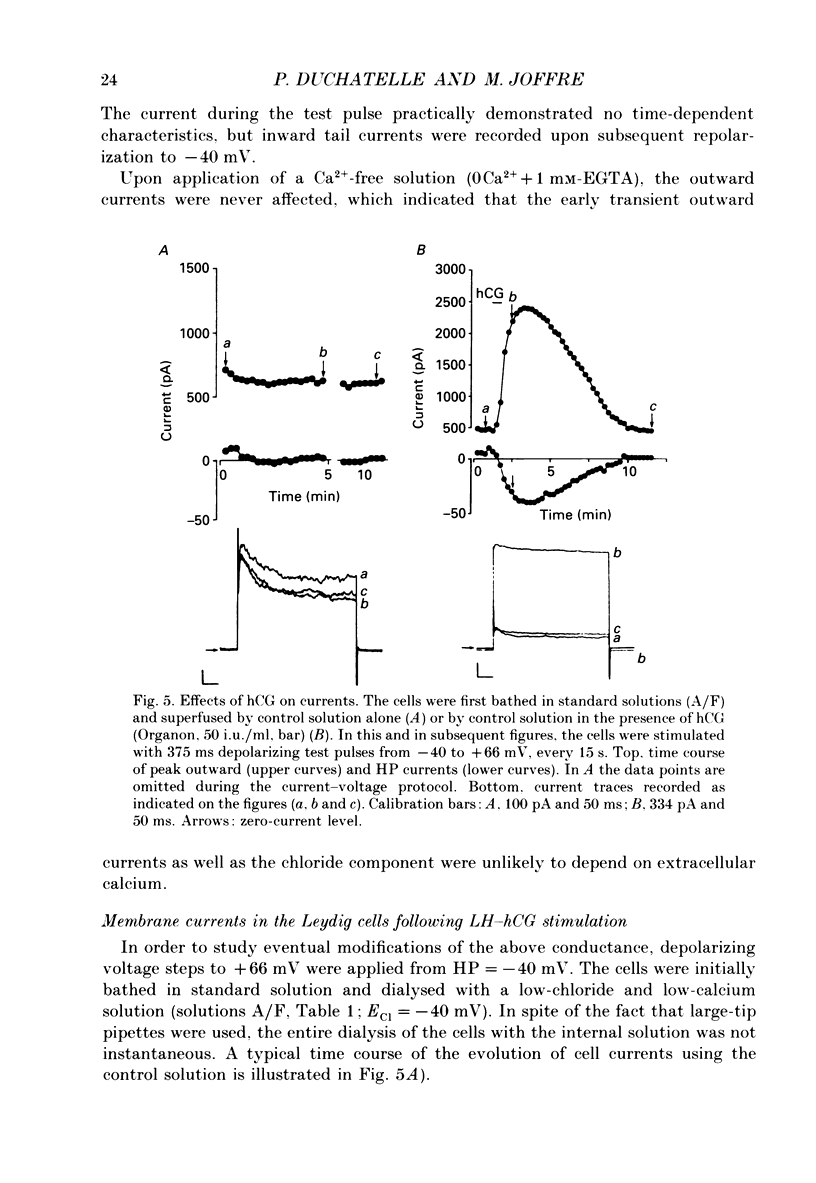
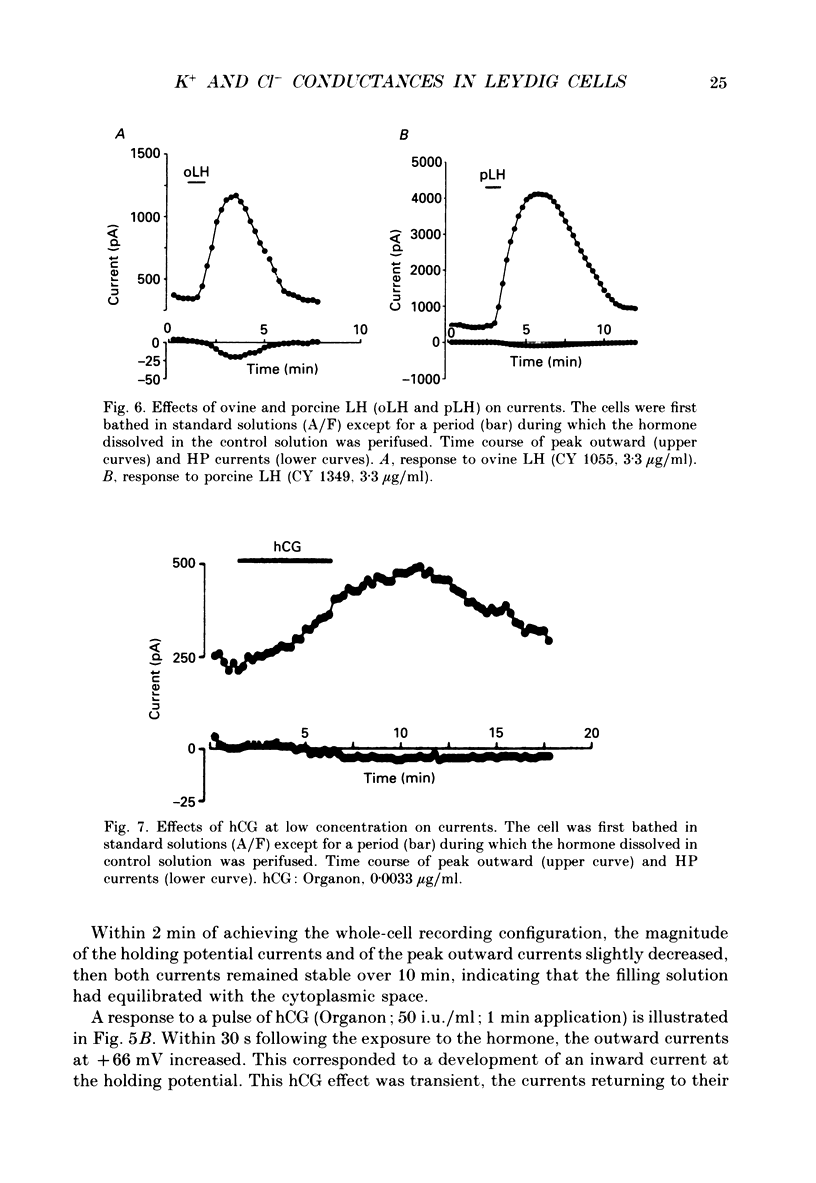
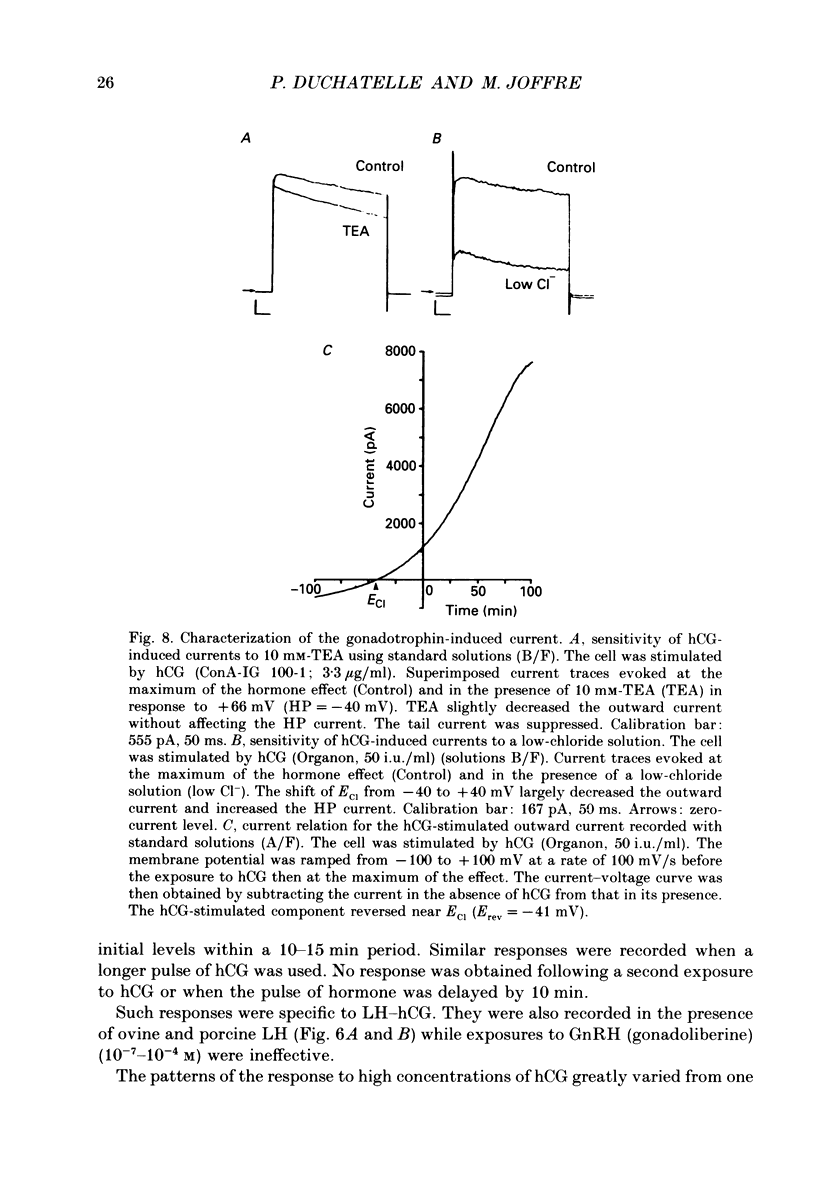






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader C. R., Bertrand D., Schlichter R. Calcium-activated chloride current in cultured sensory and parasympathetic quail neurones. J Physiol. 1987 Dec;394:125–148. doi: 10.1113/jphysiol.1987.sp016863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barish M. E. A transient calcium-dependent chloride current in the immature Xenopus oocyte. J Physiol. 1983 Sep;342:309–325. doi: 10.1113/jphysiol.1983.sp014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma M. M. Anion channels with multiple conductance levels in a mouse B lymphocyte cell line. J Physiol. 1989 Mar;410:67–90. doi: 10.1113/jphysiol.1989.sp017521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan M. D., Chandy K. G., DeCoursey T. E., Gupta S. A voltage-gated potassium channel in human T lymphocytes. J Physiol. 1985 Jan;358:197–237. doi: 10.1113/jphysiol.1985.sp015548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. H., Schulman H., Gardner P. A cAMP-regulated chloride channel in lymphocytes that is affected in cystic fibrosis. Science. 1989 Feb 3;243(4891):657–660. doi: 10.1126/science.2464852. [DOI] [PubMed] [Google Scholar]
- Cohen C. J., McCarthy R. T., Barrett P. Q., Rasmussen H. Ca channels in adrenal glomerulosa cells: K+ and angiotensin II increase T-type Ca channel current. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2412–2416. doi: 10.1073/pnas.85.7.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decoursey T. E., Chandy K. G., Gupta S., Cahalan M. D. Two types of potassium channels in murine T lymphocytes. J Gen Physiol. 1987 Mar;89(3):379–404. doi: 10.1085/jgp.89.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchatelle P., Joffre M. Ca-dependent-chloride and potassium currents in rat Leydig cells. FEBS Lett. 1987 Jun 8;217(1):11–15. doi: 10.1016/0014-5793(87)81232-2. [DOI] [PubMed] [Google Scholar]
- Dufau M. L., Catt K. J. Gonadotropin receptors and regulation of steroidogenesis in the testis and ovary. Vitam Horm. 1978;36:461–592. doi: 10.1016/s0083-6729(08)60989-9. [DOI] [PubMed] [Google Scholar]
- Dufau M. L. Endocrine regulation and communicating functions of the Leydig cell. Annu Rev Physiol. 1988;50:483–508. doi: 10.1146/annurev.ph.50.030188.002411. [DOI] [PubMed] [Google Scholar]
- Durroux T., Gallo-Payet N., Payet M. D. Three components of the calcium current in cultured glomerulosa cells from rat adrenal gland. J Physiol. 1988 Oct;404:713–729. doi: 10.1113/jphysiol.1988.sp017315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. G., Marty A. Calcium-dependent chloride currents in isolated cells from rat lacrimal glands. J Physiol. 1986 Sep;378:437–460. doi: 10.1113/jphysiol.1986.sp016229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin E. K., Sheehy P. A. Differential expression of inward and outward potassium currents in the macrophage-like cell line J774.1. J Physiol. 1985 Dec;369:475–499. doi: 10.1113/jphysiol.1985.sp015911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldez F., Murray K. J., Sepúlveda F. V., Sheppard D. N. Characterization of a phosphorylation-activated Cl-selective channel in isolated Necturus enterocytes. J Physiol. 1989 Sep;416:517–537. doi: 10.1113/jphysiol.1989.sp017775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. A., Greenwell J. R., Argent B. E. Secretin-regulated chloride channel on the apical plasma membrane of pancreatic duct cells. J Membr Biol. 1988 Oct;105(2):131–142. doi: 10.1007/BF02009166. [DOI] [PubMed] [Google Scholar]
- Guillou F., Martinat N., Combarnous Y. Rapid in vitro desensitization of the testosterone response in rat Leydig cells by sub-active concentrations of porcine luteinizing hormone. FEBS Lett. 1985 May 6;184(1):6–9. doi: 10.1016/0014-5793(85)80641-4. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harvey R. D., Hume J. R. Autonomic regulation of a chloride current in heart. Science. 1989 May 26;244(4907):983–985. doi: 10.1126/science.2543073. [DOI] [PubMed] [Google Scholar]
- Inoue I. Voltage-dependent chloride conductance of the squid axon membrane and its blockade by some disulfonic stilbene derivatives. J Gen Physiol. 1985 Apr;85(4):519–537. doi: 10.1085/jgp.85.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janszen F. H., Cooke B. A., Van Driel M. J., Van Der Molen H. J. The effect of calcium ions on testosterone production in Leydig cells from rat testis. Biochem J. 1976 Dec 15;160(3):433–437. doi: 10.1042/bj1600433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffre M., Mollard P., Régondaud P., Alix J., Poindessault J. P., Malassiné A., Gargouïl Y. M. Electrophysiological study of single Leydig cells freshly isolated from rat testis. I. Technical approach and recordings of the membrane potential in standard solution. Pflugers Arch. 1984 Jul;401(3):239–245. doi: 10.1007/BF00582590. [DOI] [PubMed] [Google Scholar]
- Joffre M., Mollard P., Régondaud P., Gargouïl Y. M. Electrophysiological study of single Leydig cells freshly isolated from rat testis. II. Effects of ionic replacements, inhibitors and human chorionic gonadotropin on a calcium activated potassium permeability. Pflugers Arch. 1984 Jul;401(3):246–253. doi: 10.1007/BF00582591. [DOI] [PubMed] [Google Scholar]
- Kawa K. Existence of calcium channels and intercellular couplings in the testosterone-secreting cells of the mouse. J Physiol. 1987 Dec;393:647–666. doi: 10.1113/jphysiol.1987.sp016846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanum A., Dufau M. L. Angiotensin II receptors and inhibitory actions in Leydig cells. J Biol Chem. 1988 Apr 15;263(11):5070–5074. [PubMed] [Google Scholar]
- Lotshaw D. P., Levitan I. B. Serotonin and forskolin modulation of a chloride conductance in cultured identified Aplysia neurons. J Neurophysiol. 1987 Nov;58(5):922–939. doi: 10.1152/jn.1987.58.5.922. [DOI] [PubMed] [Google Scholar]
- Marty A., Tan Y. P., Trautmann A. Three types of calcium-dependent channel in rat lacrimal glands. J Physiol. 1984 Dec;357:293–325. doi: 10.1113/jphysiol.1984.sp015501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga H., Yamashita N., Maruyama Y., Kojima I., Kurokawa K. Evidence for two distinct voltage-gated calcium channel currents in bovine adrenal glomerulosa cells. Biochem Biophys Res Commun. 1987 Dec 31;149(3):1049–1054. doi: 10.1016/0006-291x(87)90514-6. [DOI] [PubMed] [Google Scholar]
- Mendelson C., Dufau M., Catt K. Gonadotropin binding and stimulation of cyclic adenosine 3':5'-monophosphate and testosterone production in isolated Leydig cells. J Biol Chem. 1975 Nov 25;250(22):8818–8823. [PubMed] [Google Scholar]
- Owen D. G., Segal M., Barker J. L. A Ca-dependent Cl- conductance in cultured mouse spinal neurones. Nature. 1984 Oct 11;311(5986):567–570. doi: 10.1038/311567a0. [DOI] [PubMed] [Google Scholar]
- Payet M. D., Benabderrazik M., Gallo-Payet N. Excitation-secretion coupling: ionic currents in glomerulosa cells: effects of adrenocorticotropin and K+ channel blockers. Endocrinology. 1987 Sep;121(3):875–882. doi: 10.1210/endo-121-3-875. [DOI] [PubMed] [Google Scholar]
- Penner R., Matthews G., Neher E. Regulation of calcium influx by second messengers in rat mast cells. Nature. 1988 Aug 11;334(6182):499–504. doi: 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- Randriamampita C., Chanson M., Trautmann A. Calcium and secretagogues-induced conductances in rat exocrine pancreas. Pflugers Arch. 1988 Jan;411(1):53–57. doi: 10.1007/BF00581646. [DOI] [PubMed] [Google Scholar]
- Randriamampita C., Trautmann A. Ionic channels in murine macrophages. J Cell Biol. 1987 Aug;105(2):761–769. doi: 10.1083/jcb.105.2.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Schoppa N., Shorofsky S. R., Jow F., Nelson D. J. Voltage-gated chloride currents in cultured canine tracheal epithelial cells. J Membr Biol. 1989 Apr;108(1):73–90. doi: 10.1007/BF01870427. [DOI] [PubMed] [Google Scholar]
- Sharpe R. M., Cooper I. Relationship between the exposure of Leydig cells to factor(s) present in testicular interstitial fluid and changes in their capacity to secrete testosterone during culture or after hCG-induced desensitization. Mol Cell Endocrinol. 1987 May;51(1-2):105–114. doi: 10.1016/0303-7207(87)90124-9. [DOI] [PubMed] [Google Scholar]
- Sharpe R. M., Cooper I. Stimulatory effect of LHRH and its agonists on Leydig cell steroidogenesis in vitro. Mol Cell Endocrinol. 1982 Apr;26(1-2):141–150. doi: 10.1016/0303-7207(82)90012-0. [DOI] [PubMed] [Google Scholar]
- Sullivan M. H., Cooke B. A. The role of Ca2+ in steroidogenesis in Leydig cells. Stimulation of intracellular free Ca2+ by lutropin (LH), luliberin (LHRH) agonist and cyclic AMP. Biochem J. 1986 May 15;236(1):45–51. doi: 10.1042/bj2360045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabares L., Ureña J., López-Barneo J. Properties of calcium and potassium currents of clonal adrenocortical cells. J Gen Physiol. 1989 Mar;93(3):495–519. doi: 10.1085/jgp.93.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taleb O., Feltz P., Bossu J. L., Feltz A. Small-conductance chloride channels activated by calcium on cultured endocrine cells from mammalian pars intermedia. Pflugers Arch. 1988 Oct;412(6):641–646. doi: 10.1007/BF00583766. [DOI] [PubMed] [Google Scholar]
- Ypey D. L., Clapham D. E. Development of a delayed outward-rectifying K+ conductance in cultured mouse peritoneal macrophages. Proc Natl Acad Sci U S A. 1984 May;81(10):3083–3087. doi: 10.1073/pnas.81.10.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]


