Abstract
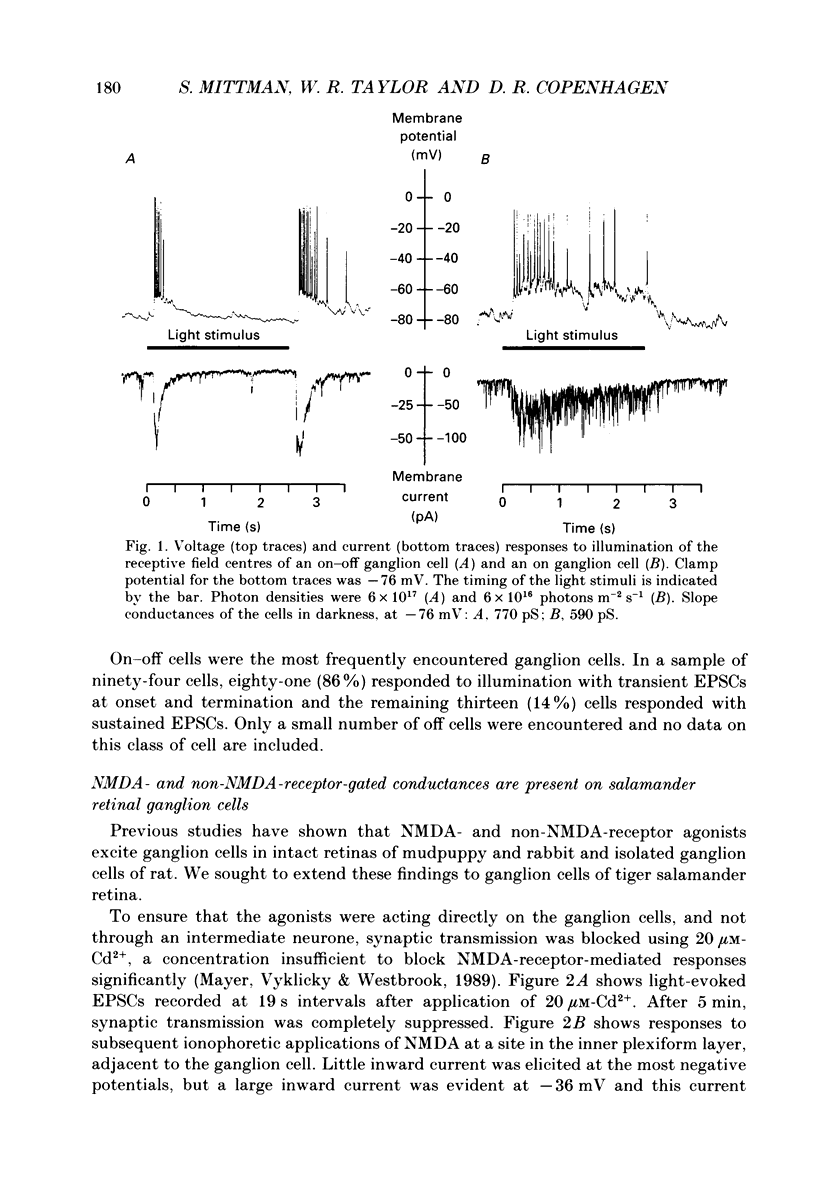
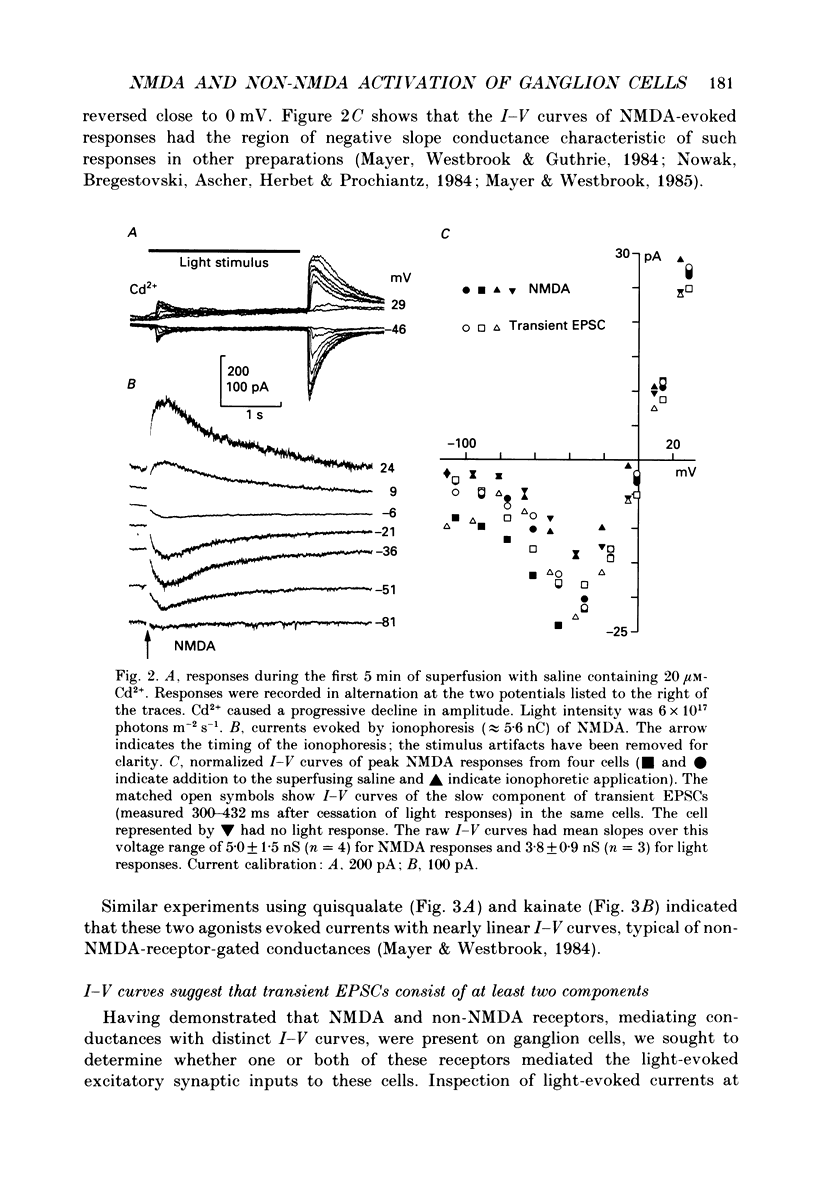
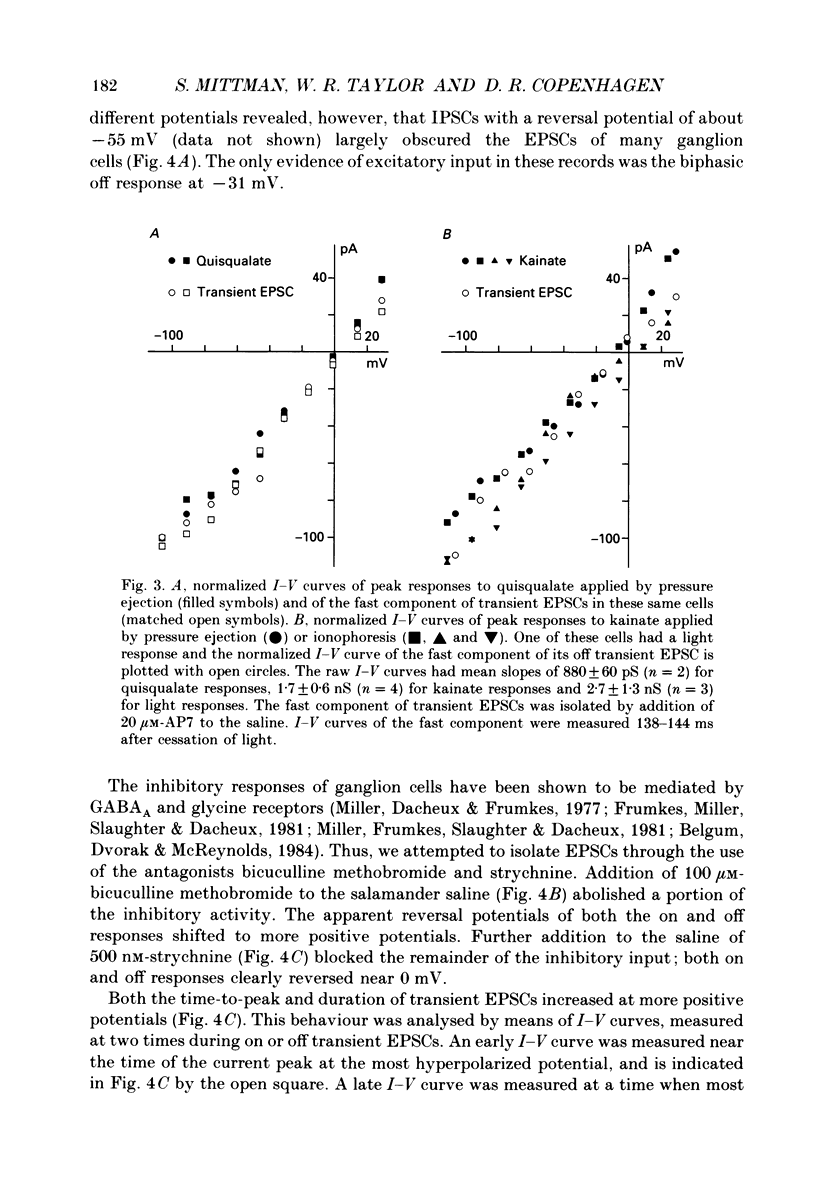
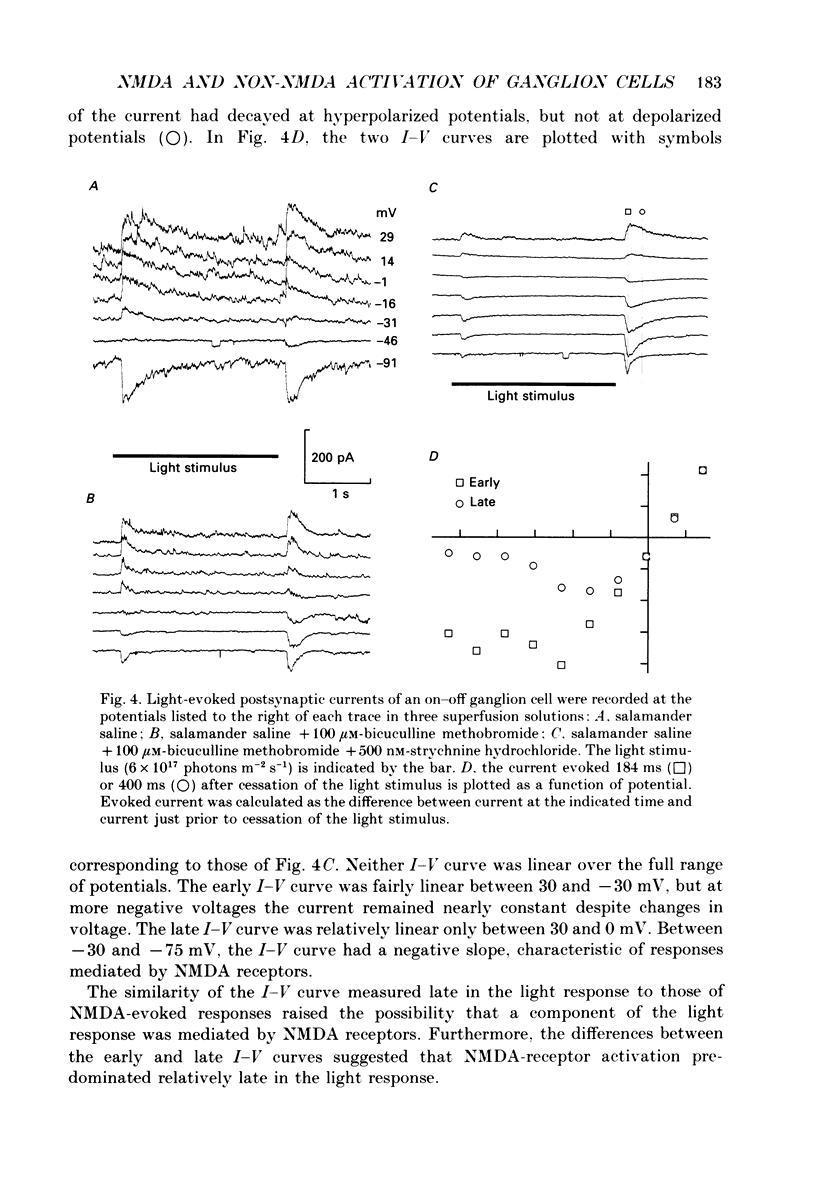
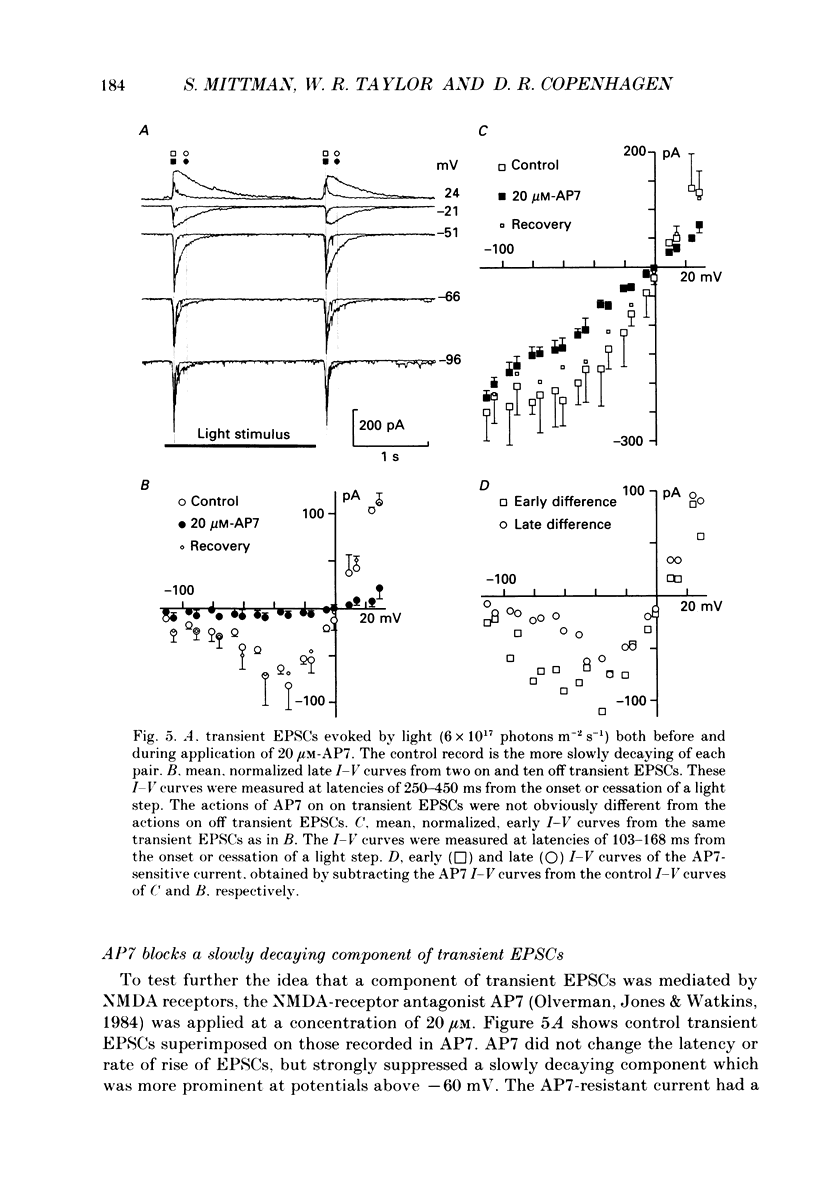
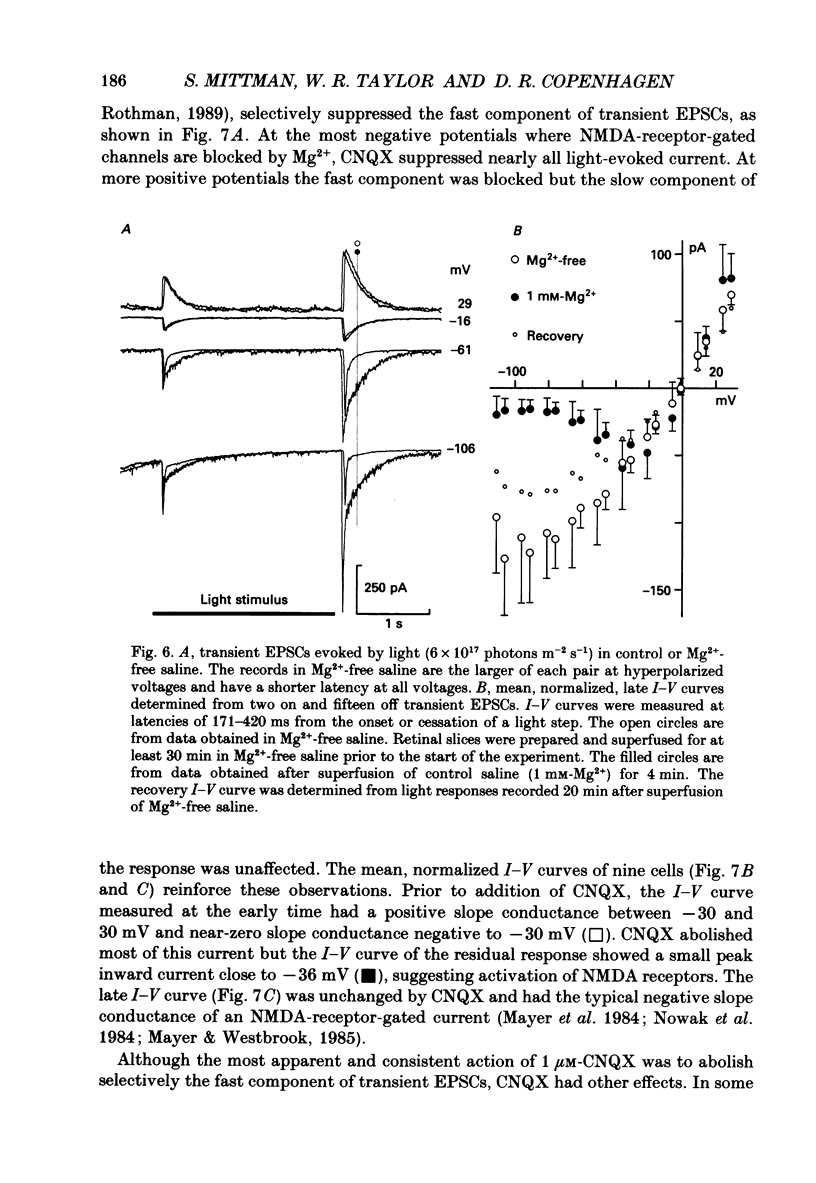
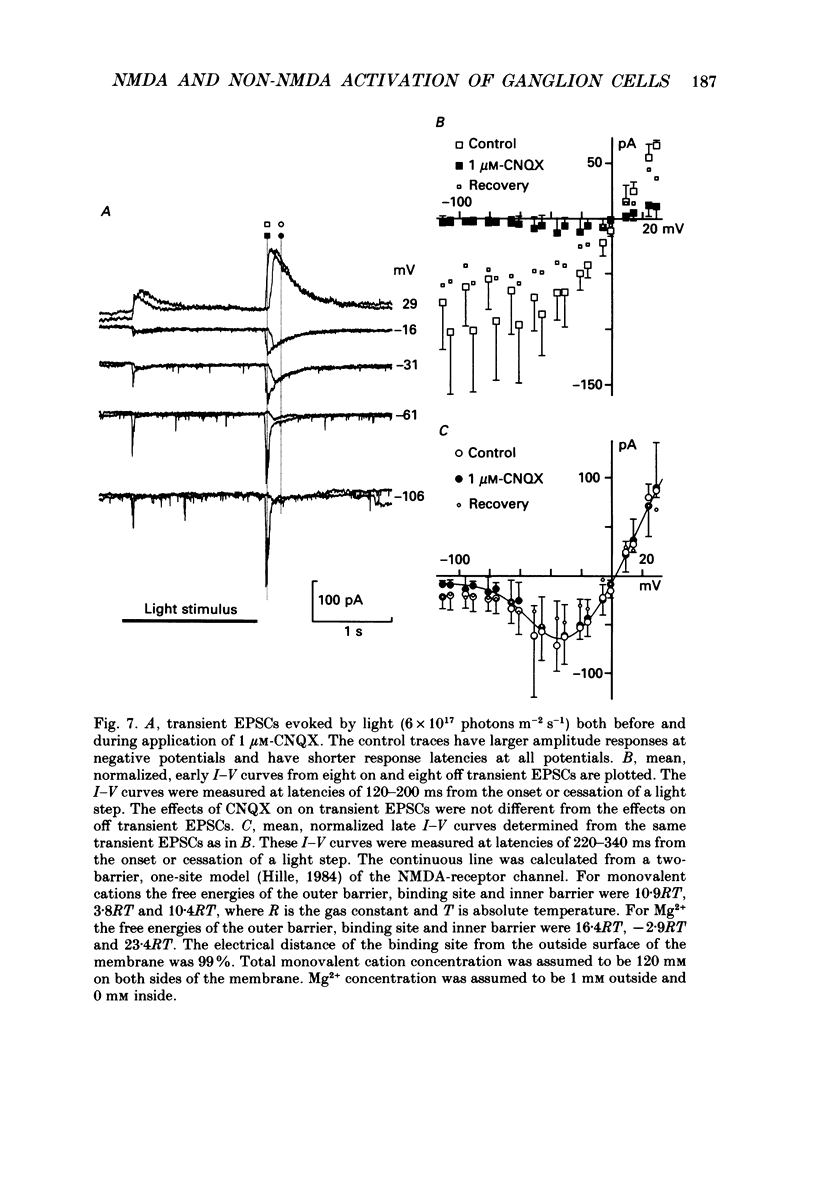
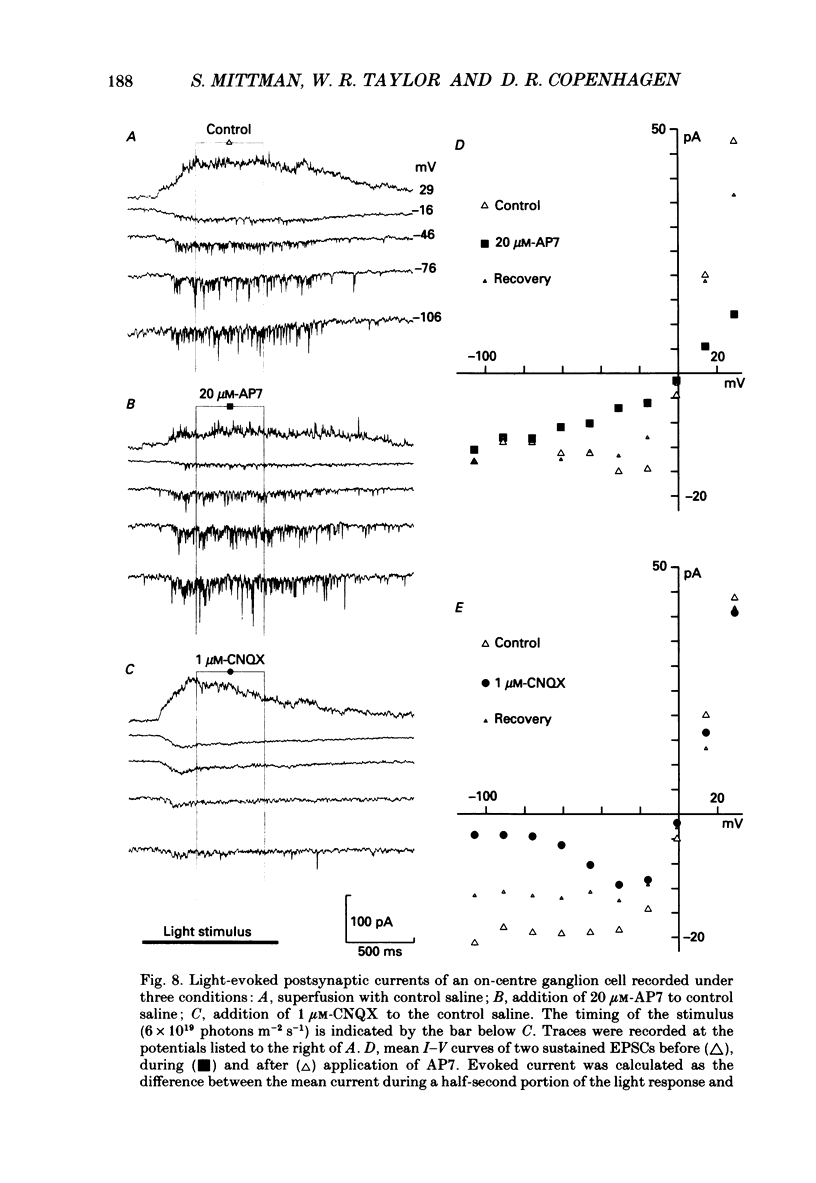
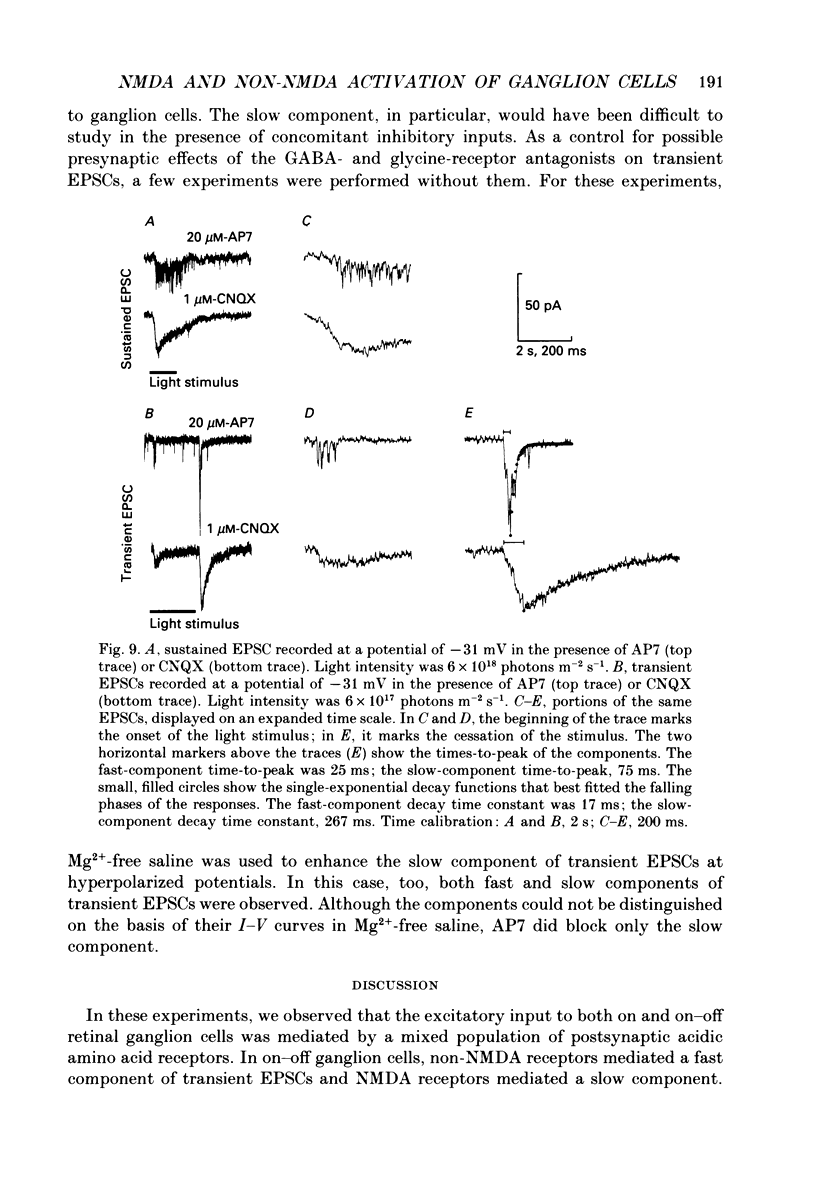
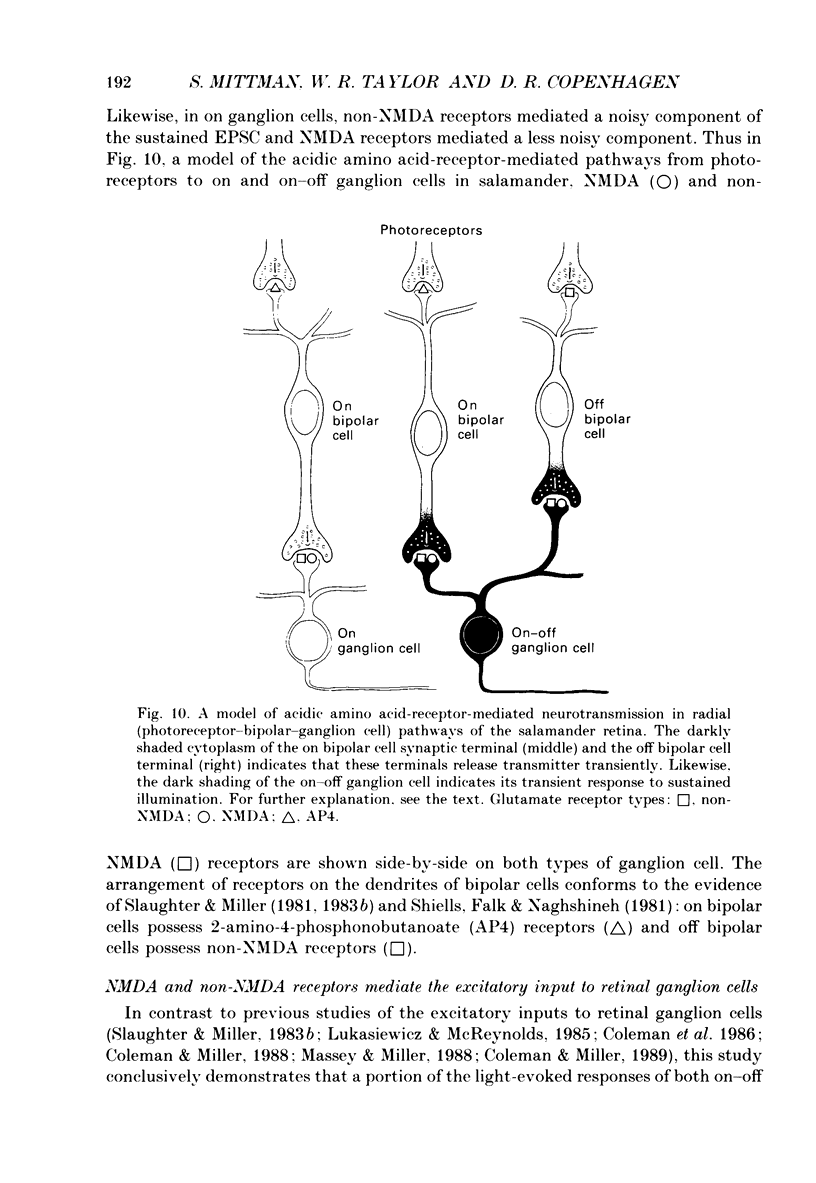
1. Cells in the ganglion cell layer of salamander retinal slices were voltage clamped using patch pipettes. Light elicited transient excitatory postsynaptic currents (EPSCs) in on-off ganglion cells and sustained EPSCs in on ganglion cells. Light-evoked inhibitory postsynaptic currents in these cells could be blocked by 100 microM-bicuculline methobromide and 500 nM-strychnine. 2. In the presence of external Cd2+, at a concentration that blocked light-evoked synaptic inputs, N-methyl-D-aspartate (NMDA) and the non-NMDA-receptor agonists, quisqualate and kainate, gated conductances in both on-off and on ganglion cells. The current-voltage (I-V) curve for the conductance elicited by NMDA had a negative slope between -40 and -70 mV and a reversal potential near 0 mV. The I-V curves for the non-NMDA-receptor-mediated conductances were nearly linear and also had reversal potentials near 0 mV. 3. I-V curves were measured at an early time point near the peak of transient EPSCs and at a later time point during the decay phase of the responses. The late I-V curve had a negative slope below -40 mV. The early I-V curve had a positive slope over the entire voltage range but the slope was greater at positive than at negative potentials. The evoked current reversed near 0 mV at both time points. 4. The region of negative slope of the late I-V curve was eliminated when Mg2+ was removed from the external saline. A slowly decaying component of transient EPSCs was eliminated in 20 microM-DL-2-amino-7-phosphonoheptanoate (AP7), an NMDA-receptor antagonist. 5. Application of 1 microM-6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), a non-NMDA-receptor antagonist at this concentration, blocked a fast component of transient EPSCs. 6. Our results demonstrate that the synaptic inputs to on-off ganglion cells have two components: a slower NMDA-receptor-mediated component having a time-to-peak of 110 +/- 45 ms and an e-fold decay time of 209 +/- 35 ms at -31 mV (mean +/- S.D., n = 5), and a faster non-NMDA-receptor-mediated component having a time-to-peak of 28 +/- 10 ms and an e-fold decay time of 43 +/- 20 ms at -31 mV (n = 8). 7. A similar analysis of sustained EPSCs of on ganglion cells showed that these currents resulted from sustained activation of both NMDA and non-NMDA receptors.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aizenman E., Frosch M. P., Lipton S. A. Responses mediated by excitatory amino acid receptors in solitary retinal ganglion cells from rat. J Physiol. 1988 Feb;396:75–91. doi: 10.1113/jphysiol.1988.sp016951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988 May;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball A. K., Dickson D. H. Displaced amacrine and ganglion cells in the newt retina. Exp Eye Res. 1983 Feb;36(2):199–213. doi: 10.1016/0014-4835(83)90006-4. [DOI] [PubMed] [Google Scholar]
- Bekkers J. M., Stevens C. F. NMDA and non-NMDA receptors are co-localized at individual excitatory synapses in cultured rat hippocampus. Nature. 1989 Sep 21;341(6239):230–233. doi: 10.1038/341230a0. [DOI] [PubMed] [Google Scholar]
- Belgum J. H., Dvorak D. R., McReynolds J. S. Strychnine blocks transient but not sustained inhibition in mudpuppy retinal ganglion cells. J Physiol. 1984 Sep;354:273–286. doi: 10.1113/jphysiol.1984.sp015375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman P. A., Massey S. C., Miller R. F. Kynurenic acid distinguishes kainate and quisqualate receptors in the vertebrate retina. Brain Res. 1986 Aug 27;381(1):172–175. doi: 10.1016/0006-8993(86)90708-0. [DOI] [PubMed] [Google Scholar]
- Coleman P. A., Miller R. F. Do N-methyl-D-aspartate receptors mediate synaptic responses in the mudpuppy retina? J Neurosci. 1988 Dec;8(12):4728–4733. doi: 10.1523/JNEUROSCI.08-12-04728.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman P. A., Miller R. F. Kainate receptor-mediated synaptic currents in mudpuppy inner retinal neurons reduced by D-O-phosphoserine. J Neurophysiol. 1989 Aug;62(2):495–500. doi: 10.1152/jn.1989.62.2.495. [DOI] [PubMed] [Google Scholar]
- Dale N., Roberts A. Dual-component amino-acid-mediated synaptic potentials: excitatory drive for swimming in Xenopus embryos. J Physiol. 1985 Jun;363:35–59. doi: 10.1113/jphysiol.1985.sp015694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe I. D., Westbrook G. L. Slow excitatory postsynaptic currents mediated by N-methyl-D-aspartate receptors on cultured mouse central neurones. J Physiol. 1988 Feb;396:515–533. doi: 10.1113/jphysiol.1988.sp016975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frumkes T. E., Miller R. F., Slaughter M., Dacheux R. F. Physiological and pharmacological basis of GABA and glycine action on neurons of mudpuppy retina. III. Amacrine-mediated inhibitory influences on ganglion cell receptive-field organization: a model. J Neurophysiol. 1981 Apr;45(4):783–804. doi: 10.1152/jn.1981.45.4.783. [DOI] [PubMed] [Google Scholar]
- Hoffman R., Gross L. The modulation contrast microscope. Nature. 1975 Apr 17;254(5501):586–588. doi: 10.1038/254586a0. [DOI] [PubMed] [Google Scholar]
- Honoré T., Davies S. N., Drejer J., Fletcher E. J., Jacobsen P., Lodge D., Nielsen F. E. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988 Aug 5;241(4866):701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz P. D., McReynolds J. S. Synaptic transmission at N-methyl-D-aspartate receptors in the proximal retina of the mudpuppy. J Physiol. 1985 Oct;367:99–115. doi: 10.1113/jphysiol.1985.sp015816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasiewicz P., Werblin F. A slowly inactivating potassium current truncates spike activity in ganglion cells of the tiger salamander retina. J Neurosci. 1988 Dec;8(12):4470–4481. doi: 10.1523/JNEUROSCI.08-12-04470.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire G., Lukasiewicz P., Werblin F. Amacrine cell interactions underlying the response to change in the tiger salamander retina. J Neurosci. 1989 Feb;9(2):726–735. doi: 10.1523/JNEUROSCI.09-02-00726.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiafava P. L., Torre V. The responses of amacrine cells to light and intracellularly applied currents. J Physiol. 1978 Mar;276:83–102. doi: 10.1113/jphysiol.1978.sp012221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey S. C., Miller R. F. Glutamate receptors of ganglion cells in the rabbit retina: evidence for glutamate as a bipolar cell transmitter. J Physiol. 1988 Nov;405:635–655. doi: 10.1113/jphysiol.1988.sp017353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr, Westbrook G. L. Modulation of excitatory amino acid receptors by group IIB metal cations in cultured mouse hippocampal neurones. J Physiol. 1989 Aug;415:329–350. doi: 10.1113/jphysiol.1989.sp017724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. Mixed-agonist action of excitatory amino acids on mouse spinal cord neurones under voltage clamp. J Physiol. 1984 Sep;354:29–53. doi: 10.1113/jphysiol.1984.sp015360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J Physiol. 1987 Dec;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The action of N-methyl-D-aspartic acid on mouse spinal neurones in culture. J Physiol. 1985 Apr;361:65–90. doi: 10.1113/jphysiol.1985.sp015633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. F., Dacheux R. F., Frumkes T. E. Amacrine cells in Necturus retina: evidence for independent gamma-aminobutyric acid- and glycine-releasing neurons. Science. 1977 Nov 18;198(4318):748–750. doi: 10.1126/science.910159. [DOI] [PubMed] [Google Scholar]
- Miller R. F., Frumkes T. E., Slaughter M., Dacheux R. F. Physiological and pharmacological basis of GABA and glycine action on neurons of mudpuppy retina. II. Amacrine and ganglion cells. J Neurophysiol. 1981 Apr;45(4):764–782. doi: 10.1152/jn.1981.45.4.764. [DOI] [PubMed] [Google Scholar]
- Mittman S., Flaming D. G., Copenhagen D. R., Belgum J. H. Bubble pressure measurement of micropipet tip outer diameter. J Neurosci Methods. 1987 Dec;22(2):161–166. doi: 10.1016/0165-0270(87)90010-0. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Olverman H. J., Jones A. W., Watkins J. C. L-glutamate has higher affinity than other amino acids for [3H]-D-AP5 binding sites in rat brain membranes. Nature. 1984 Feb 2;307(5950):460–462. doi: 10.1038/307460a0. [DOI] [PubMed] [Google Scholar]
- Perry V. H. The ganglion cell layer of the retina of the rat: a Golgi study. Proc R Soc Lond B Biol Sci. 1979 May 23;204(1156):363–375. doi: 10.1098/rspb.1979.0033. [DOI] [PubMed] [Google Scholar]
- Shiells R. A., Falk G., Naghshineh S. Action of glutamate and aspartate analogues on rod horizontal and bipolar cells. Nature. 1981 Dec 10;294(5841):592–594. doi: 10.1038/294592a0. [DOI] [PubMed] [Google Scholar]
- Slaughter M. M., Miller R. F. 2-amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science. 1981 Jan 9;211(4478):182–185. doi: 10.1126/science.6255566. [DOI] [PubMed] [Google Scholar]
- Slaughter M. M., Miller R. F. Bipolar cells in the mudpuppy retina use an excitatory amino acid neurotransmitter. Nature. 1983 Jun 9;303(5917):537–538. doi: 10.1038/303537a0. [DOI] [PubMed] [Google Scholar]
- Slaughter M. M., Miller R. F. The role of excitatory amino acid transmitters in the mudpuppy retina: an analysis with kainic acid and N-methyl aspartate. J Neurosci. 1983 Aug;3(8):1701–1711. doi: 10.1523/JNEUROSCI.03-08-01701.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. E., Delaney R. K., Powell R. E. Retinal ganglion cell response to axotomy in the regenerating visual system of the newt (Triturus viridescens): an ultrastructural morphometric analysis. Exp Neurol. 1978 Nov;62(2):444–462. doi: 10.1016/0014-4886(78)90067-5. [DOI] [PubMed] [Google Scholar]
- Werblin F. S. Transmission along and between rods in the tiger salamander retina. J Physiol. 1978 Jul;280:449–470. doi: 10.1113/jphysiol.1978.sp012394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigström H., Gustafsson B., Huang Y. Y. A synaptic potential following single volleys in the hippocampal CA1 region possibly involved in the induction of long-lasting potentiation. Acta Physiol Scand. 1985 Jul;124(3):475–478. doi: 10.1111/j.1748-1716.1985.tb07685.x. [DOI] [PubMed] [Google Scholar]
- Wu S. M. Synaptic connections between neurons in living slices of the larval tiger salamander retina. J Neurosci Methods. 1987 Jun;20(2):139–149. doi: 10.1016/0165-0270(87)90046-x. [DOI] [PubMed] [Google Scholar]
- Wunk D. F., Werblin F. S. Synaptic inputs to the ganglion cells in the tiger salamander retina. J Gen Physiol. 1979 Mar;73(3):265–286. doi: 10.1085/jgp.73.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. A., Dubinsky J. M., Rothman S. M. Quantitative physiological characterization of a quinoxalinedione non-NMDA receptor antagonist. J Neurosci. 1989 Sep;9(9):3230–3236. doi: 10.1523/JNEUROSCI.09-09-03230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


