Abstract
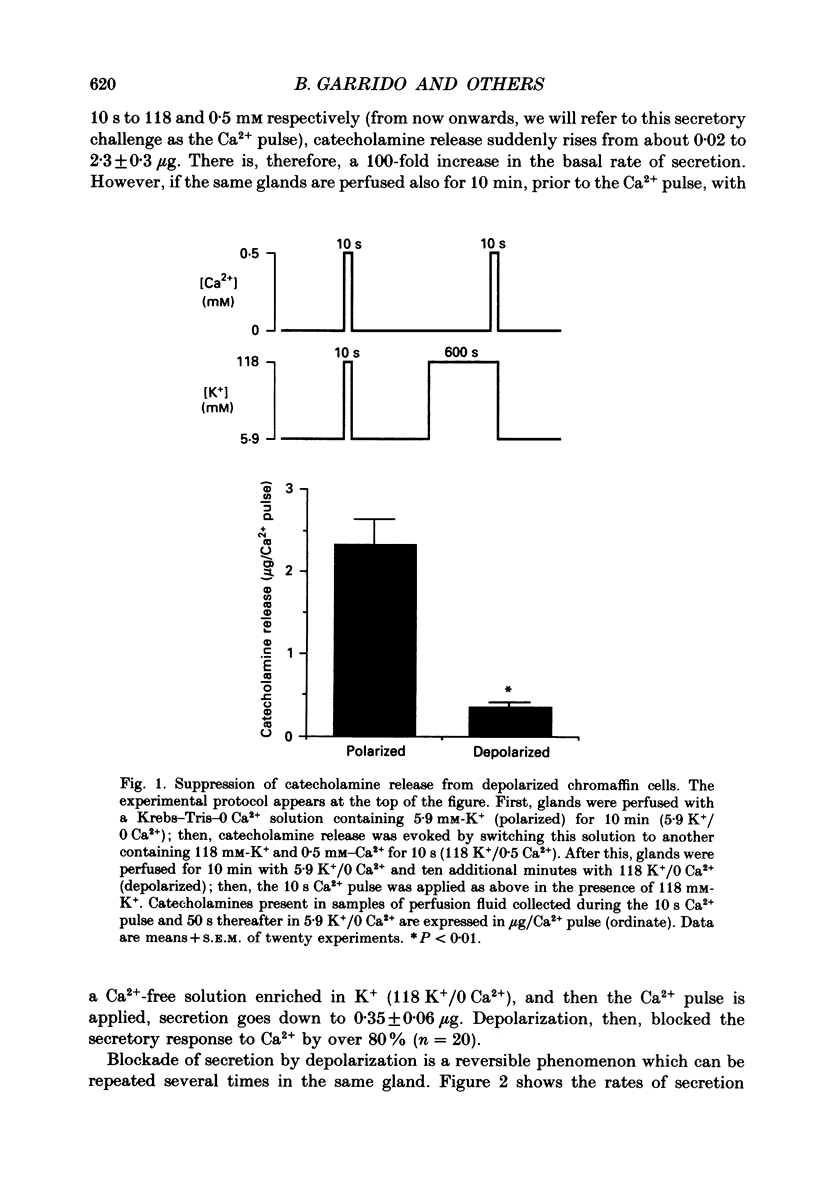
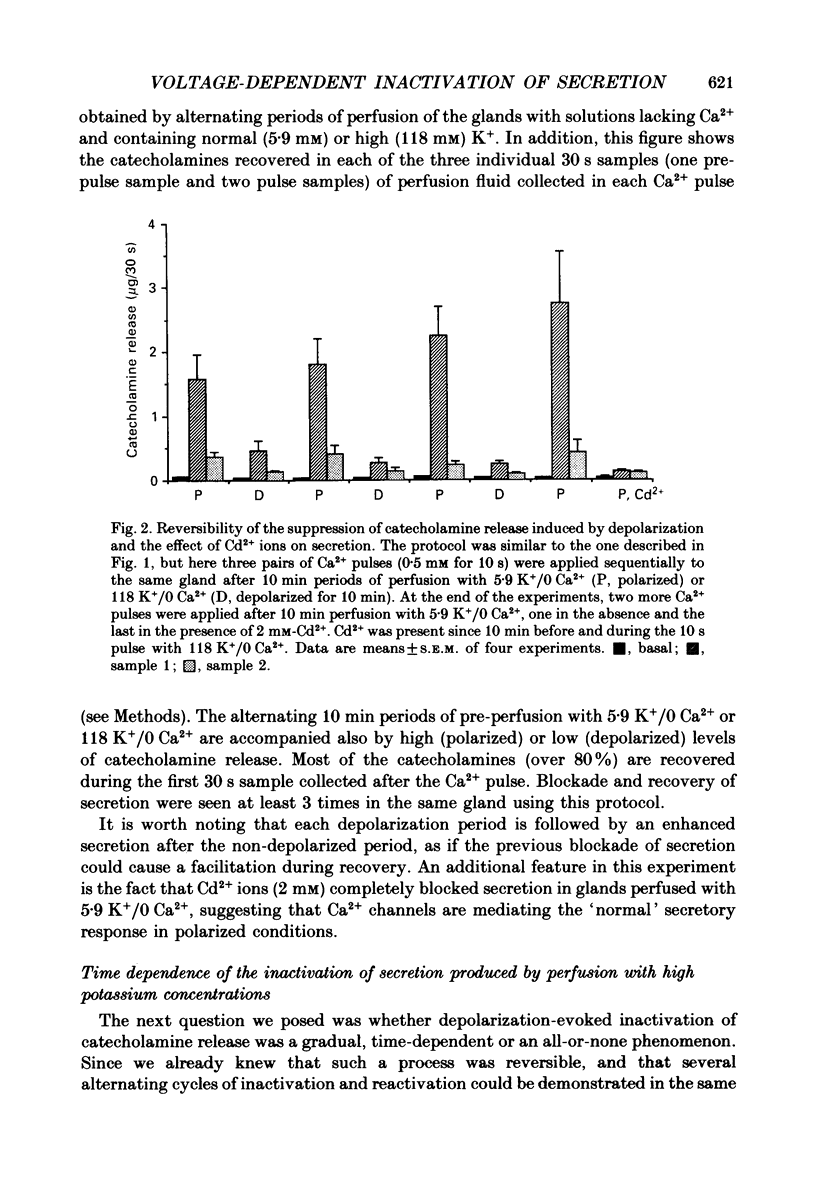
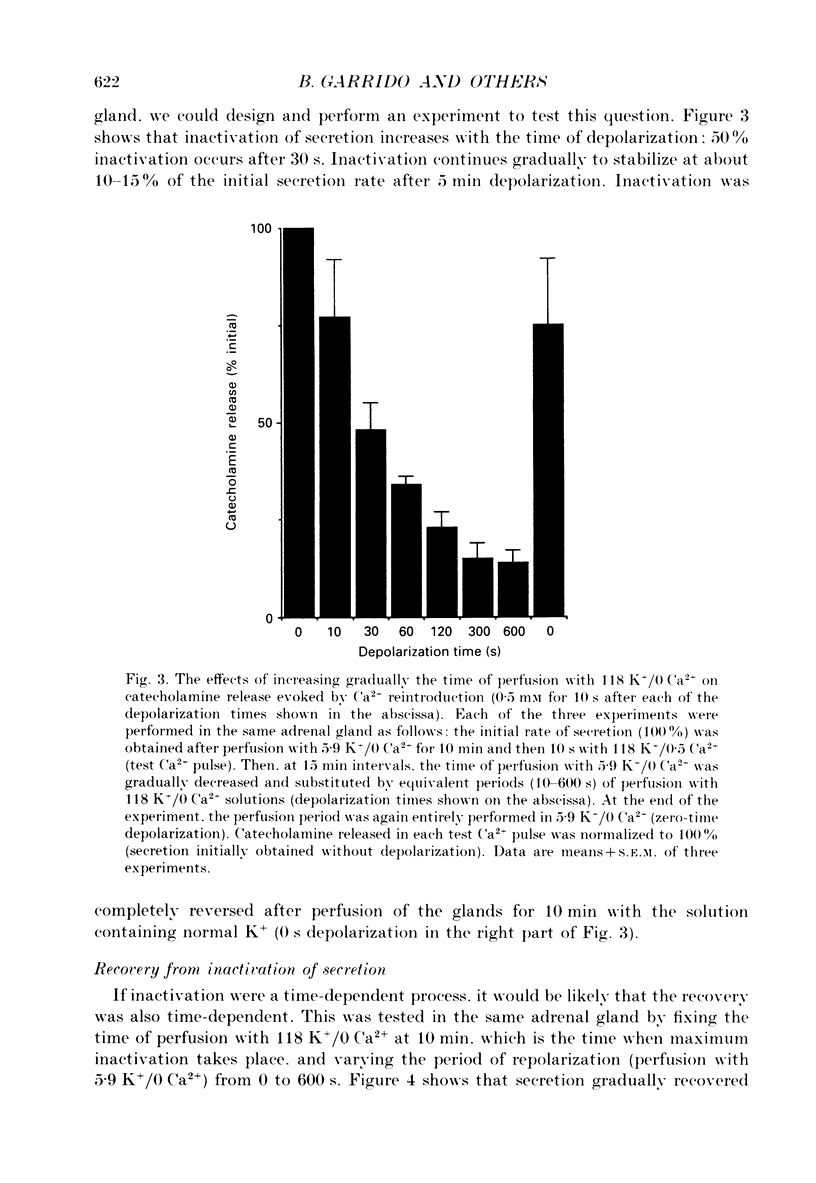
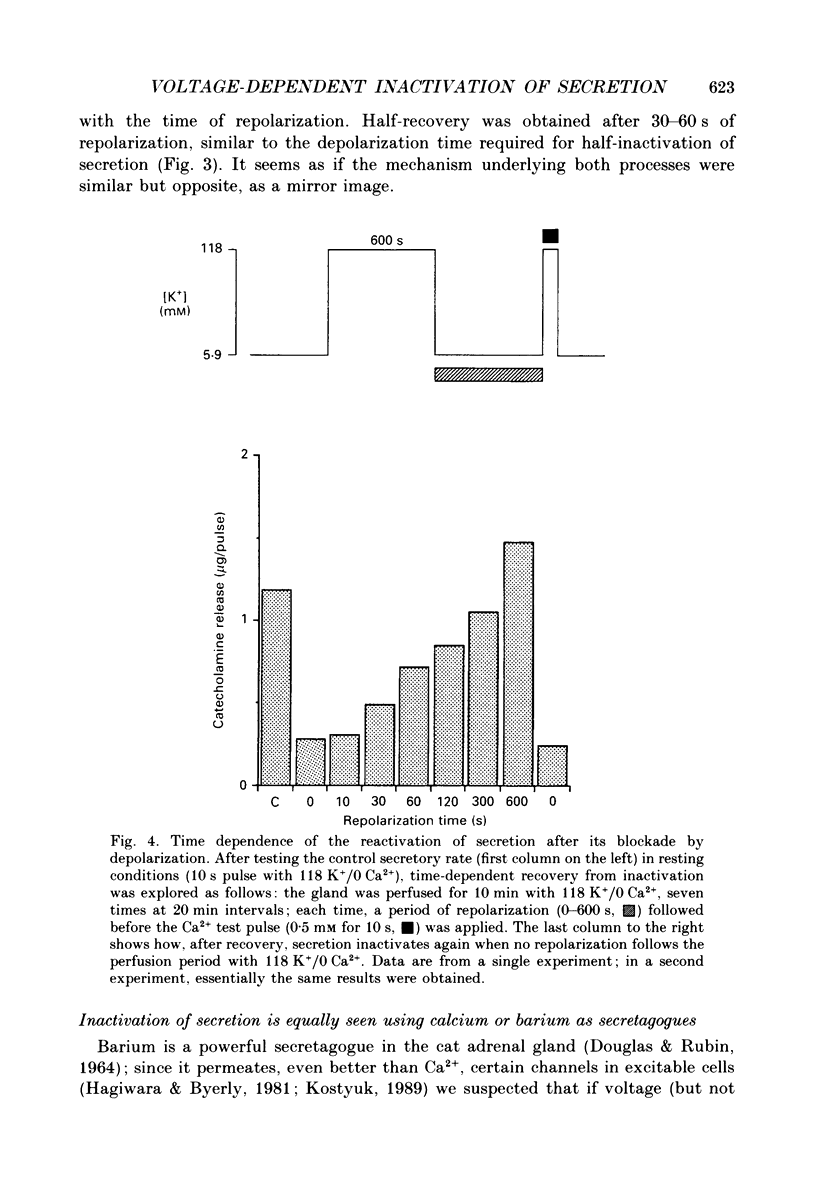
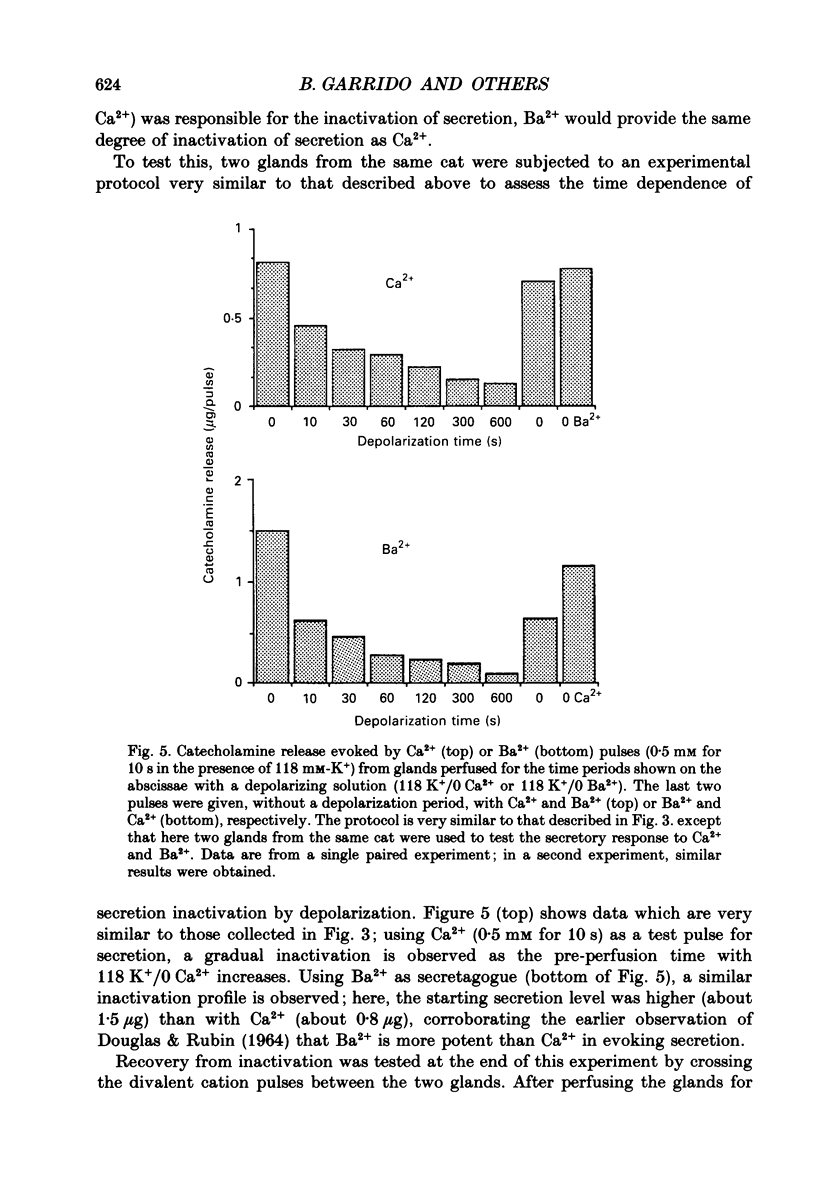
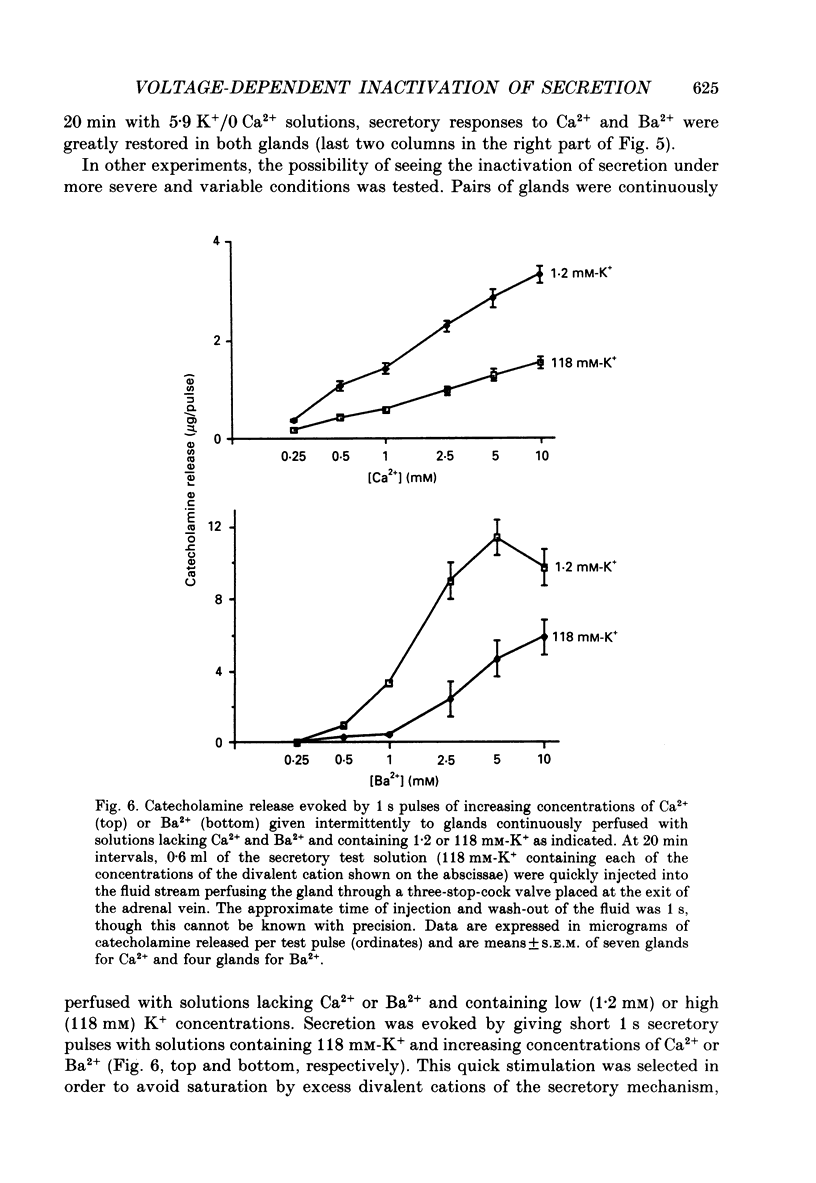
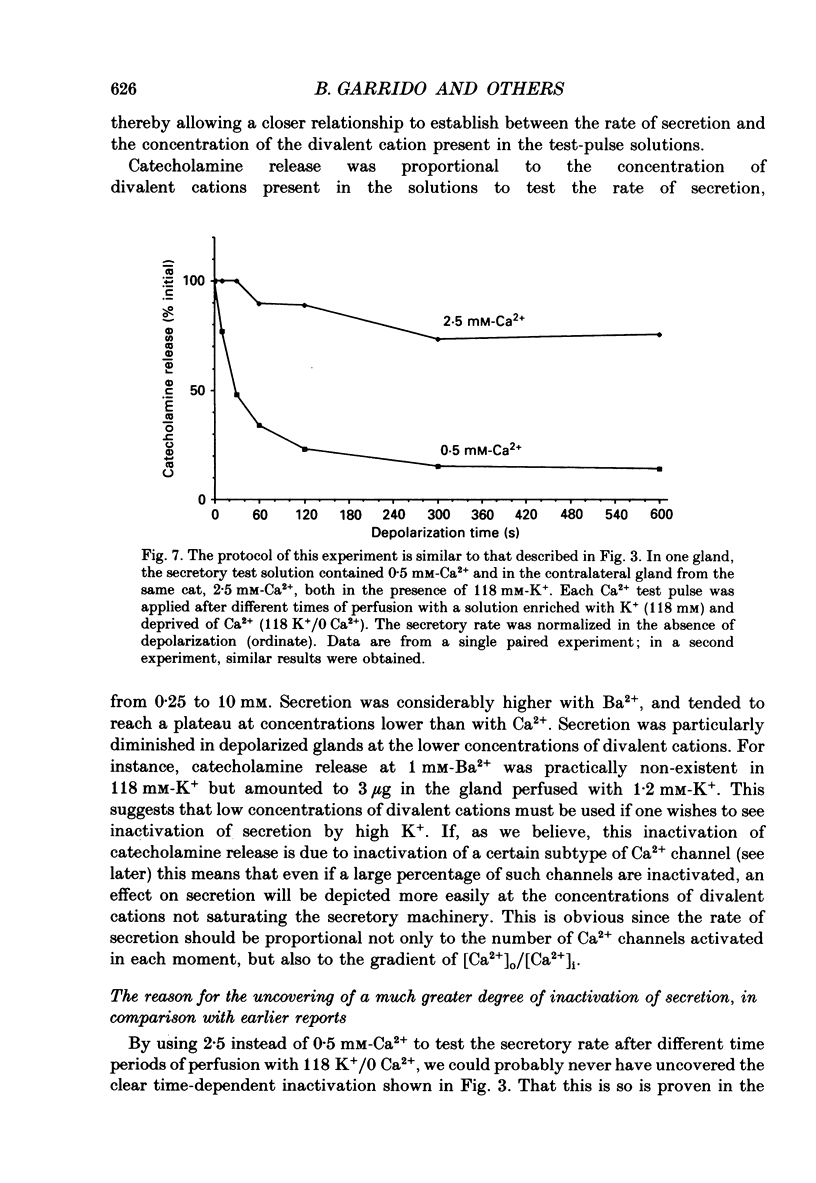
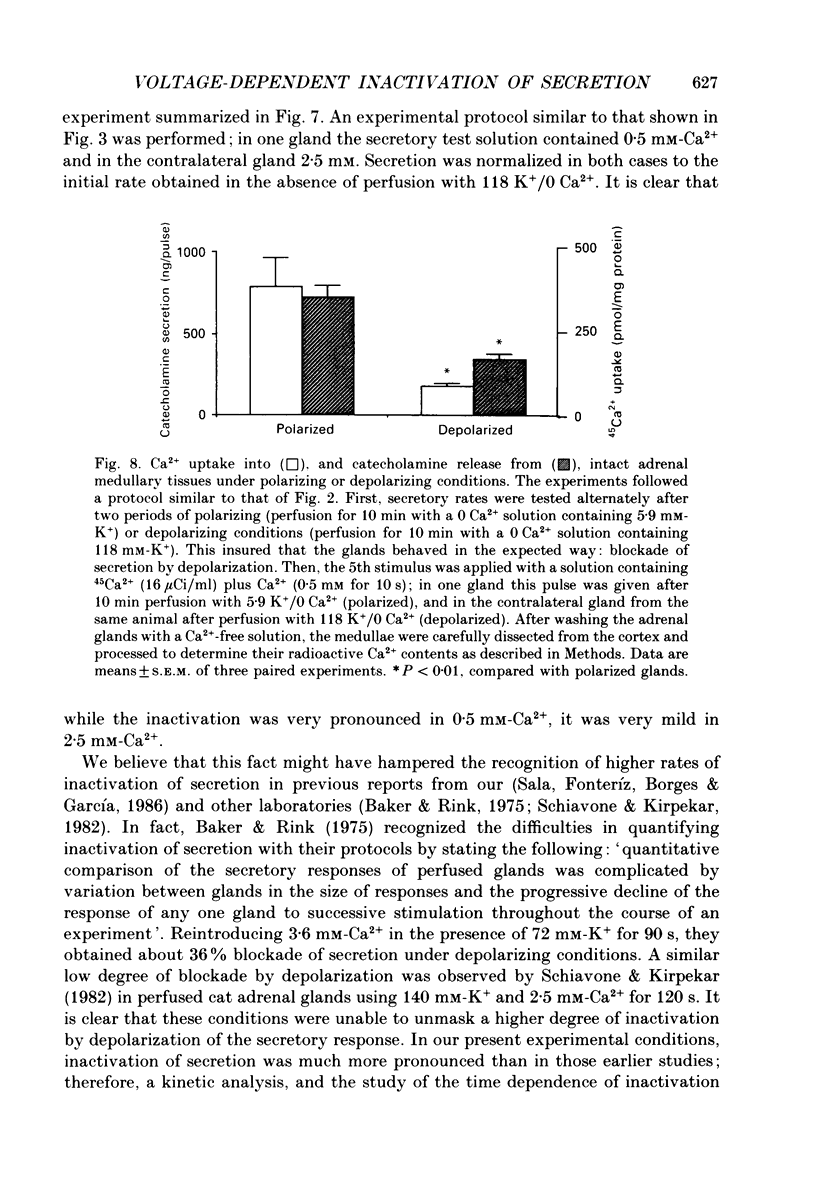
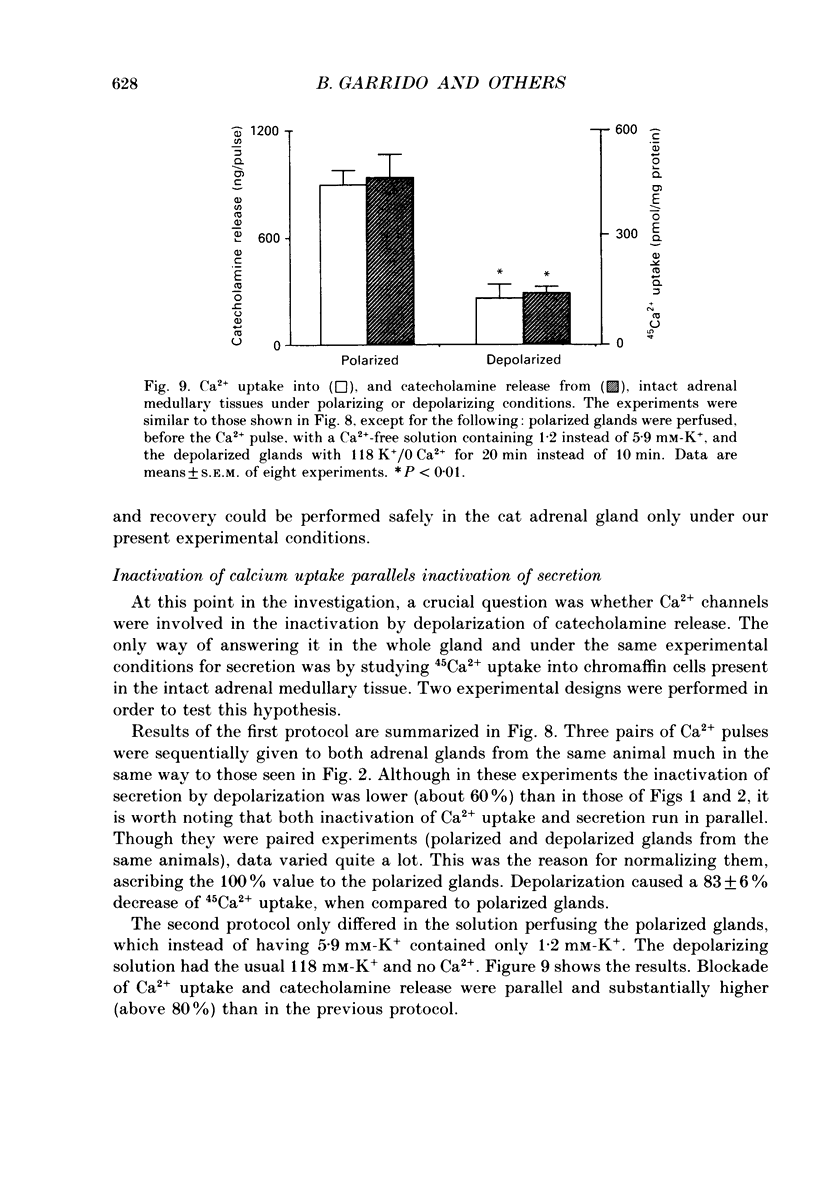
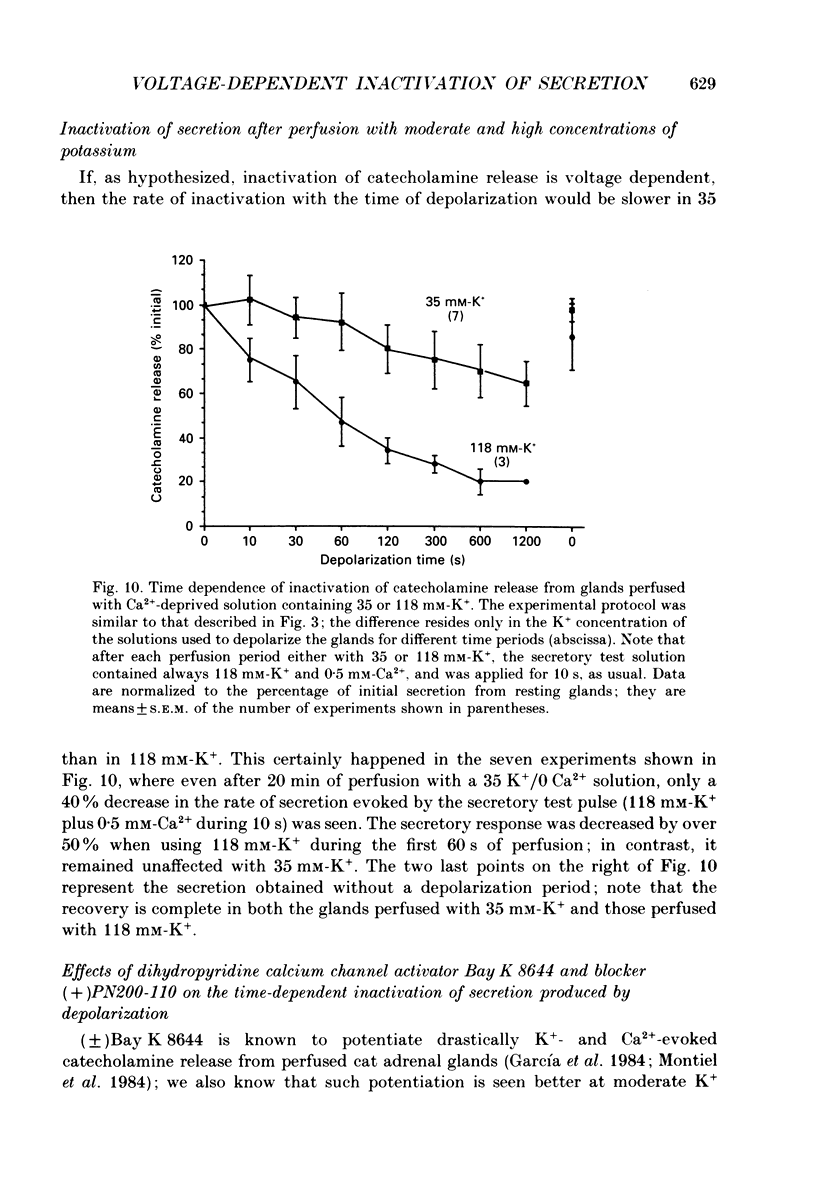
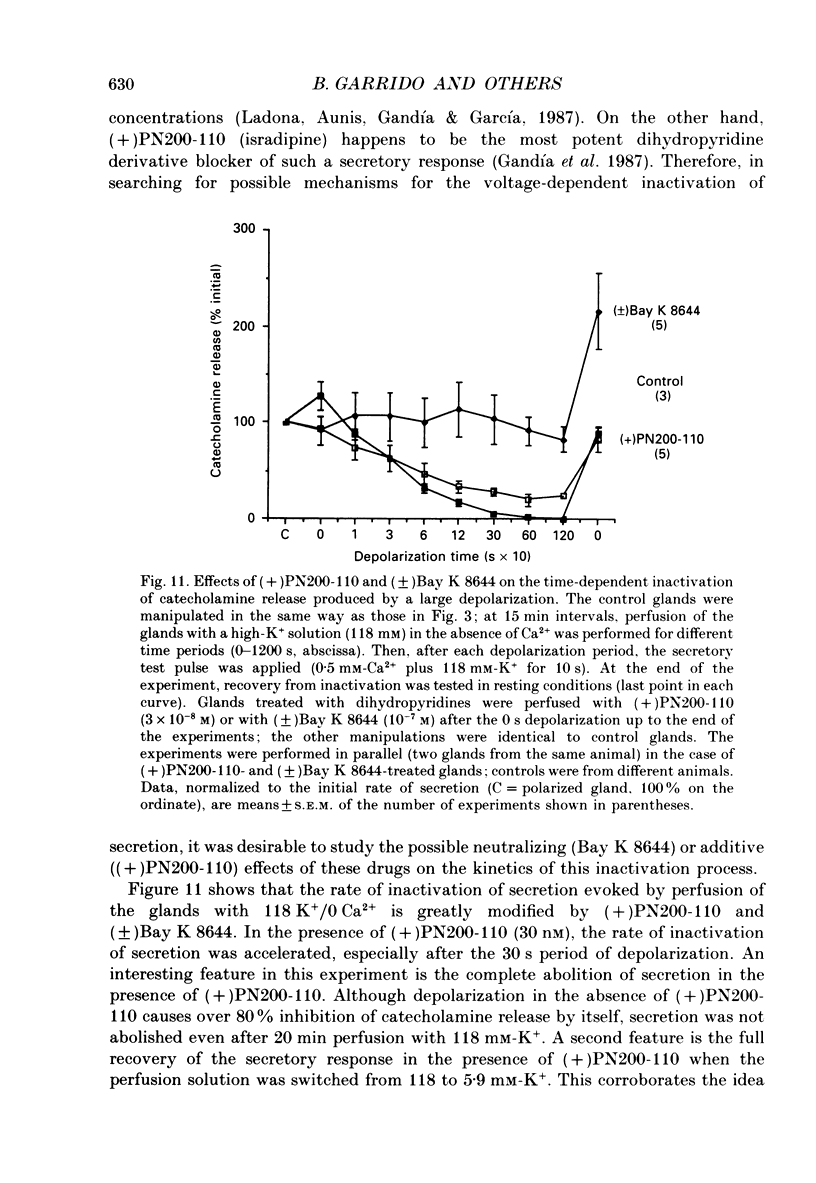
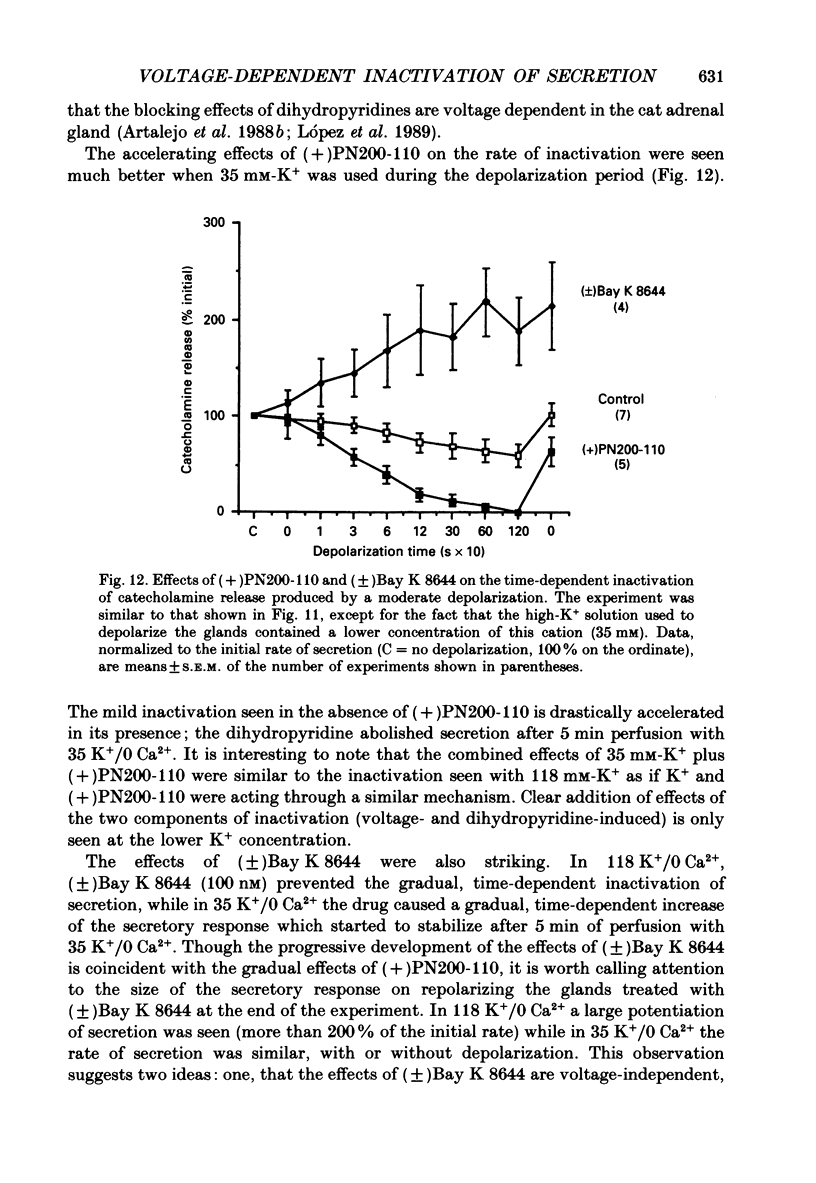
1. Inactivation by voltage changes of 45Ca2+ uptake into and catecholamine release from cat adrenal glands perfused at a high rate (4 ml/min) at 37 degrees C with oxygenated Krebs-Tris solution has been studied. Experimental conditions were selected so that adrenal medullary chromaffin cells were depolarized for different time periods and with various K+ concentrations in the absence of Ca2+, prior to the application of 0.5 mM-Ca2+ for 10 s in the presence of 118 mM-K+ to test the rate of secretion (the 'Ca2+ pulse'). 2. Application of the Ca2+ pulse after perfusion with 5.9 mM-K+ led to a 100-fold increase of the basal rate of secretion. However, if the Ca2+ pulse was preceded by a 10 min period of perfusion with 118 mM-K+, the secretory response was decreased by over 80%. 3. Inactivation of secretion starts 10-30 s after high-K+ perfusion and is completed 2-5 min thereafter. Inactivation is readily reversed by perfusing the glands with normal K(+)-containing solution; the recovery phenomenon is also gradual and time-dependent, starting 30 s after repolarization and ending 300 s thereafter. 4. The rate of inactivation is much slower at 35 than at 118 mM-K+, suggesting that the process is strongly dependent on voltage. 5. Like catecholamine release, Ca2+ uptake into adrenal medullary chromaffin cells is inactivated in a voltage-dependent manner. This, together with the fact that Cd2+ blocked secretion completely and inactivation was seen equally using Ca2+ or Ba2+ as secretagogues, suggests that inactivation of a certain class of voltage-dependent Ca2+ channels is responsible for the blockade of secretion. Such channels must be slowly inactivated by voltage and highly sensitive to dihydropyridines, since (+)PN200-110 (an L-type Ca2+ channel blocker) enhanced the rate of inactivation and (+/-)Bay K 8644 (an L-type Ca2+ channel activator) prevented it, indicating that they might belong to L-subtype Ca2+ channels. 6. The effects of (+/-)Bay K 8644 (100 nM) were seen on both the voltage and time dependence of inactivation. At a moderate depolarization (35 mM-K+), the drug prevented inactivation and caused potentiation of secretion which developed gradually; at strong depolarizations (118 mM-K+), Bay K 8644 prevented the time-dependent development of inactivation. (+)PN200-110 (30 nM) did not suddenly decrease catecholamine release at the earlier times of depolarization; what the drug did was to accelerate the normal rate of inactivation induced by depolarization.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albino A. P., Le Strange R., Oliff A. I., Furth M. E., Old L. J. Transforming ras genes from human melanoma: a manifestation of tumour heterogeneity? Nature. 1984 Mar 1;308(5954):69–72. doi: 10.1038/308069a0. [DOI] [PubMed] [Google Scholar]
- Artalejo C. R., Bader M. F., Aunis D., García A. G. Inactivation of the early calcium uptake and noradrenaline release evoked by potassium in cultured chromaffin cells. Biochem Biophys Res Commun. 1986 Jan 14;134(1):1–7. doi: 10.1016/0006-291x(86)90518-8. [DOI] [PubMed] [Google Scholar]
- Artalejo C. R., García A. G., Aunis D. Chromaffin cell calcium channel kinetics measured isotopically through fast calcium, strontium, and barium fluxes. J Biol Chem. 1987 Jan 15;262(2):915–926. [PubMed] [Google Scholar]
- Baker P. F., Rink T. J. Catecholamine release from bovine adrenal medulla in response to maintained depolarization. J Physiol. 1975 Dec;253(2):593–620. doi: 10.1113/jphysiol.1975.sp011209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesta J. J., Palmero M., Hidalgo M. J., Gutierrez L. M., Reig J. A., Viniegra S., Garcia A. G. Separate binding and functional sites for omega-conotoxin and nitrendipine suggest two types of calcium channels in bovine chromaffin cells. J Neurochem. 1989 Oct;53(4):1050–1056. doi: 10.1111/j.1471-4159.1989.tb07394.x. [DOI] [PubMed] [Google Scholar]
- Borges R., Ballesta J. J., García A. G. M2 muscarinoceptor-associated ionophore at the cat adrenal medulla. Biochem Biophys Res Commun. 1987 Apr 29;144(2):965–972. doi: 10.1016/s0006-291x(87)80058-x. [DOI] [PubMed] [Google Scholar]
- Brehm P., Eckert R. Calcium entry leads to inactivation of calcium channel in Paramecium. Science. 1978 Dec 15;202(4373):1203–1206. doi: 10.1126/science.103199. [DOI] [PubMed] [Google Scholar]
- Callewaert G., Hanbauer I., Morad M. Modulation of calcium channels in cardiac and neuronal cells by an endogenous peptide. Science. 1989 Feb 3;243(4891):663–666. doi: 10.1126/science.2536955. [DOI] [PubMed] [Google Scholar]
- Castillo C. J., Fonteríz R. I., López M. G., Rosenheck K., García A. G. (+)-PN200-110 and ouabain binding sites in purified bovine adrenomedullary plasma membranes and chromaffin cells. J Neurochem. 1989 Nov;53(5):1442–1449. doi: 10.1111/j.1471-4159.1989.tb08536.x. [DOI] [PubMed] [Google Scholar]
- Chad J. E., Eckert R. An enzymatic mechanism for calcium current inactivation in dialysed Helix neurones. J Physiol. 1986 Sep;378:31–51. doi: 10.1113/jphysiol.1986.sp016206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D. E., Neher E. Trifluoperazine reduces inward ionic currents and secretion by separate mechanisms in bovine chromaffin cells. J Physiol. 1984 Aug;353:541–564. doi: 10.1113/jphysiol.1984.sp015350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. On the mode of action of acetylcholine in evoking adrenal medullary secretion: increased uptake of calcium during the secretory response. J Physiol. 1962 Aug;162:385–392. doi: 10.1113/jphysiol.1962.sp006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. THE EFFECTS OF ALKALINE EARTHS AND OTHER DIVALENT CATIONS ON ADRENAL MEDULLARY SECRETION. J Physiol. 1964 Dec;175:231–241. doi: 10.1113/jphysiol.1964.sp007514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonteriz R. I., Gandia L., Lopez M. G., Artalejo C. R., García A. G. Dihydropyridine chirality at the chromaffin cell calcium channel. Brain Res. 1987 Apr 7;408(1-2):359–362. doi: 10.1016/0006-8993(87)90405-7. [DOI] [PubMed] [Google Scholar]
- Fox A. P. Voltage-dependent inactivation of a calcium channel. Proc Natl Acad Sci U S A. 1981 Feb;78(2):953–956. doi: 10.1073/pnas.78.2.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandía L., López M. G., Fonteríz R. I., Artalejo C. R., García A. G. Relative sensitivities of chromaffin cell calcium channels to organic and inorganic calcium antagonists. Neurosci Lett. 1987 Jun 26;77(3):333–338. doi: 10.1016/0304-3940(87)90523-4. [DOI] [PubMed] [Google Scholar]
- Garcia A. G., Hernandez M., Horga J. F., Sanchez-Garcia P. On the release of catecholamines and dopamine-beta-hydroxylase evoked by ouabain in the perfused cat adrenal gland. Br J Pharmacol. 1980 Mar;68(3):571–583. doi: 10.1111/j.1476-5381.1980.tb14573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hirning L. D., Fox A. P., McCleskey E. W., Olivera B. M., Thayer S. A., Miller R. J., Tsien R. W. Dominant role of N-type Ca2+ channels in evoked release of norepinephrine from sympathetic neurons. Science. 1988 Jan 1;239(4835):57–61. doi: 10.1126/science.2447647. [DOI] [PubMed] [Google Scholar]
- Holz R. W., Senter R. A., Frye R. A. Relationship between Ca2+ uptake and catecholamine secretion in primary dissociated cultures of adrenal medulla. J Neurochem. 1982 Sep;39(3):635–646. doi: 10.1111/j.1471-4159.1982.tb07940.x. [DOI] [PubMed] [Google Scholar]
- Hoshi T., Rothlein J., Smith S. J. Facilitation of Ca2+-channel currents in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5871–5875. doi: 10.1073/pnas.81.18.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T., Smith S. J. Large depolarization induces long openings of voltage-dependent calcium channels in adrenal chromaffin cells. J Neurosci. 1987 Feb;7(2):571–580. doi: 10.1523/JNEUROSCI.07-02-00571.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K., Kanno T. Influences of extracellular calcium and potassium concentrations on adrenaline release and membrane potential in the perfused adrenal medulla of the rat. Jpn J Physiol. 1978;28(3):275–289. doi: 10.2170/jjphysiol.28.275. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Hescheler J., Mieskes G., Trautwein W. The protein-specific phosphatase 1 antagonizes the beta-adrenergic increase of the cardiac Ca current. Pflugers Arch. 1986 Oct;407(4):461–463. doi: 10.1007/BF00652635. [DOI] [PubMed] [Google Scholar]
- Kilpatrick D. L., Slepetis R. J., Corcoran J. J., Kirshner N. Calcium uptake and catecholamine secretion by cultured bovine adrenal medulla cells. J Neurochem. 1982 Feb;38(2):427–435. doi: 10.1111/j.1471-4159.1982.tb08647.x. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G. Diversity of calcium ion channels in cellular membranes. Neuroscience. 1989;28(2):253–261. doi: 10.1016/0306-4522(89)90177-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ladona M. G., Aunis D., Gandía L., García A. G. Dihydropyridine modulation of the chromaffin cell secretory response. J Neurochem. 1987 Feb;48(2):483–490. doi: 10.1111/j.1471-4159.1987.tb04118.x. [DOI] [PubMed] [Google Scholar]
- López M. G., Moro M. A., Castillo C. F., Artalejo C. R., García A. G. Variable, voltage-dependent, blocking effects of nitrendipine, verapamil, diltiazem, cinnarizine and cadmium on adrenomedullary secretion. Br J Pharmacol. 1989 Mar;96(3):725–731. doi: 10.1111/j.1476-5381.1989.tb11874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montiel C., Artalejo A. R., García A. G. Effects of the novel dihydropyridine BAY-K-8644 on adrenomedullary catecholamine release evoked by calcium reintroduction. Biochem Biophys Res Commun. 1984 May 16;120(3):851–857. doi: 10.1016/s0006-291x(84)80185-0. [DOI] [PubMed] [Google Scholar]
- Rosario L. M., Soria B., Feuerstein G., Pollard H. B. Voltage-sensitive calcium flux into bovine chromaffin cells occurs through dihydropyridine-sensitive and dihydropyridine- and omega-conotoxin-insensitive pathways. Neuroscience. 1989;29(3):735–747. doi: 10.1016/0306-4522(89)90145-0. [DOI] [PubMed] [Google Scholar]
- Sala F., Fonteriz R. I., Borges R., García A. G. Inactivation of potassium-evoked adrenomedullary catecholamine release in the presence of calcium, strontium or BAY-K-8644. FEBS Lett. 1986 Feb 3;196(1):34–38. doi: 10.1016/0014-5793(86)80209-5. [DOI] [PubMed] [Google Scholar]
- Schiavone M. T., Kirpekar S. M. Inactivation of secretory responses to potassium and nicotine in the cat adrenal medulla. J Pharmacol Exp Ther. 1982 Dec;223(3):743–749. [PubMed] [Google Scholar]
- Shellenberger M. K., Gordon J. H. A rapid, simplified procedure for simultaneous assay of norepinephrine, dopamine, and 5-hydroxytryptamine from discrete brain areas. Anal Biochem. 1971 Feb;39(2):356–372. doi: 10.1016/0003-2697(71)90426-x. [DOI] [PubMed] [Google Scholar]
- Tillotson D. Inactivation of Ca conductance dependent on entry of Ca ions in molluscan neurons. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1497–1500. doi: 10.1073/pnas.76.3.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W. Calcium channels in excitable cell membranes. Annu Rev Physiol. 1983;45:341–358. doi: 10.1146/annurev.ph.45.030183.002013. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Lipscombe D., Madison D. V., Bley K. R., Fox A. P. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988 Oct;11(10):431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- Wada A., Takara H., Izumi F., Kobayashi H., Yanagihara N. Influx of 22Na through acetylcholine receptor-associated Na channels: relationship between 22Na influx, 45Ca influx and secretion of catecholamines in cultured bovine adrenal medulla cells. Neuroscience. 1985 May;15(1):283–292. doi: 10.1016/0306-4522(85)90135-6. [DOI] [PubMed] [Google Scholar]


