Abstract
1. It was previously noticed that mouse soleus, but not extensor digitorum longus (EDL) muscles, suffer fibre damage at the onset of voluntary wheel-running without further injuries thereafter. 2. In CBA/J mice trained continuously for 5 months and rested for periods of 1, 2, 3, 4 and 5 weeks acute muscle damage was found in soleus 7 days after the resumption of wheel-running. On single cross-sections damage was present on average in 8.7 +/- 3.5% (mean +/- S.D., n = 15) of the fibres, but only in 0.47 +/- 0.21% (n = 9) and 1.3 +/- 1.1% (n = 4) in control animals rested for 0-6 weeks after continuous running or in untrained controls. 3. Repeated muscle damage occurred when mice exercised for 4 days at intervals of 21-25 days, and after thirteen running episodes within 12 months marked changes in soleus, but not EDL muscles, were present. In cross-sections the total number of muscle fibre profiles was significantly larger in soleus of intermittent runners (768 +/- 68, n = 6; P less than 0.05), compared to continuous runners (676 +/- 54, n = 3) and sedentary animals (683 +/- 33, n = 4). This is probably due to incomplete repair which results in 'split fibres'. 4. At the same time total muscle fibre cross-sectional area was significantly elevated in intermittent runners (P less than 0.05), mainly due to increase in fibre diameters. Net cross-sectional areas were 0.59 +/- 0.069 mm2 (n = 6) in intermittent, 0.53 +/- 0.076 mm2 (n = 3) in continuous runners and 0.46 +/- 0.031 mm2 (n = 3) in sedentary controls. 5. Tetanic and twitch force were also significantly elevated in soleus of intermittent runners while the ratio force/area remained the same. 6. There was an increase in the proportion of type I fibres in soleus from 75 +/- 0.9% (n = 4) in untrained controls to 90 +/- 4.4% (n = 6; P less than 0.05) in intermittent runners and 81 +/- 5.6% (n = 3; n.s.) in continuous runners. 7. Resistance to block of synaptic transmission in soleus was significantly higher in intermittent runners for two levels of curare, indicating enhanced safety margins. 8. EDL muscles in intermittent runners were not different from sedentary controls in any of the parameters studied. In particular, muscle fibres with signs of previous damage (split fibres, central nuclei) were rare (on average 0.5-0.6%) and equally frequent in all experimental groups.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
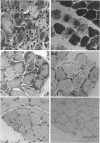
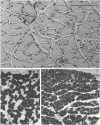
PDF



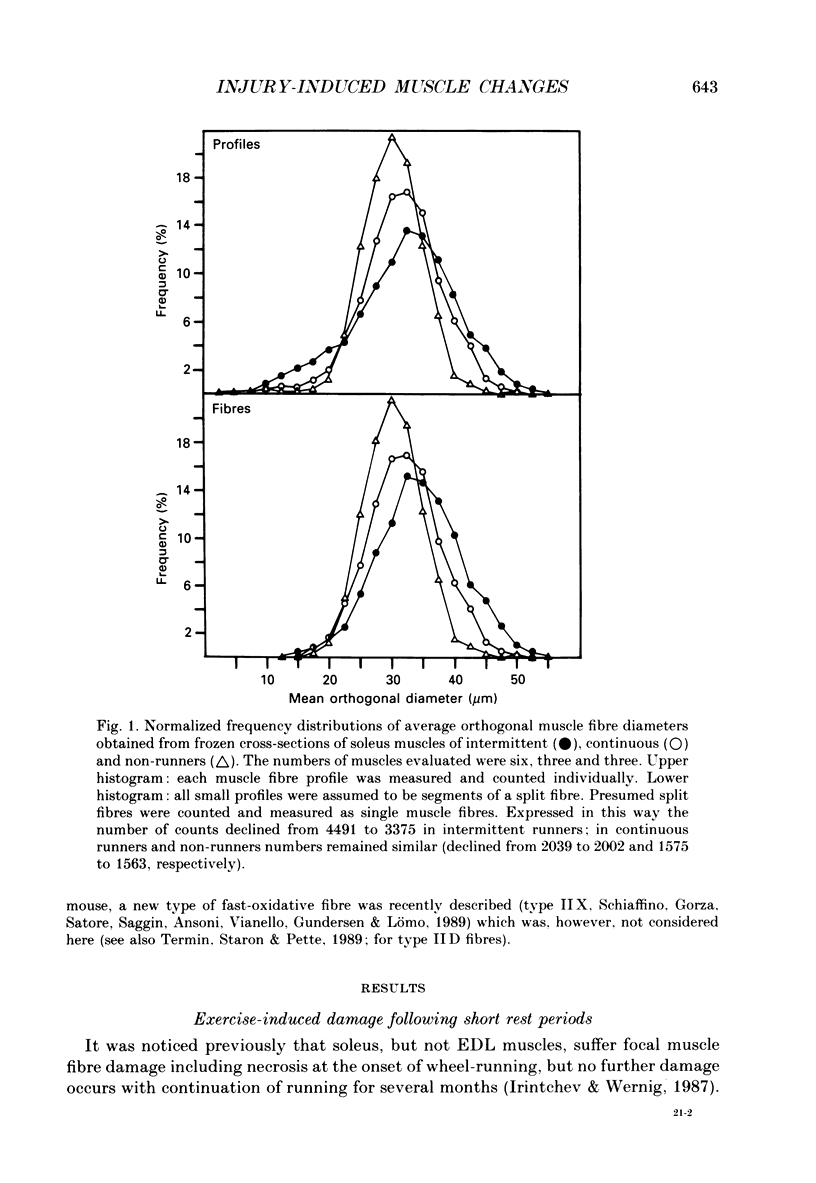

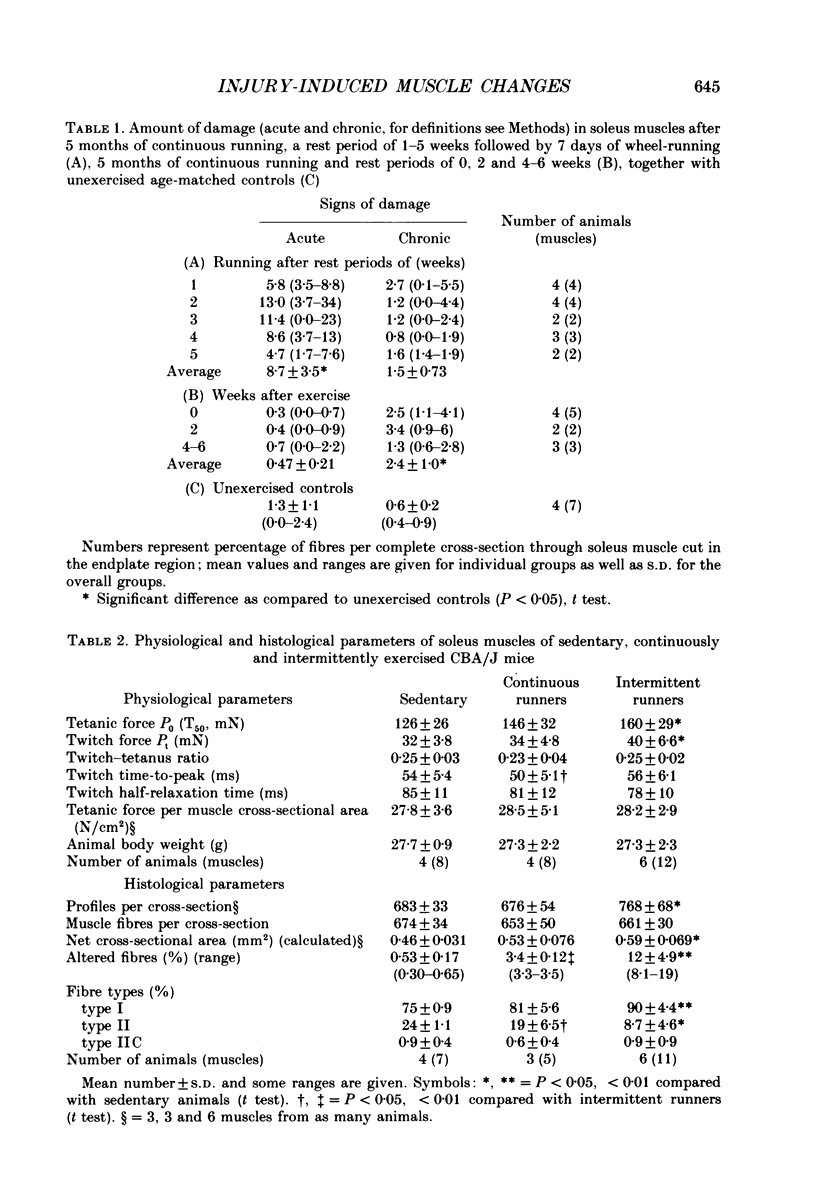

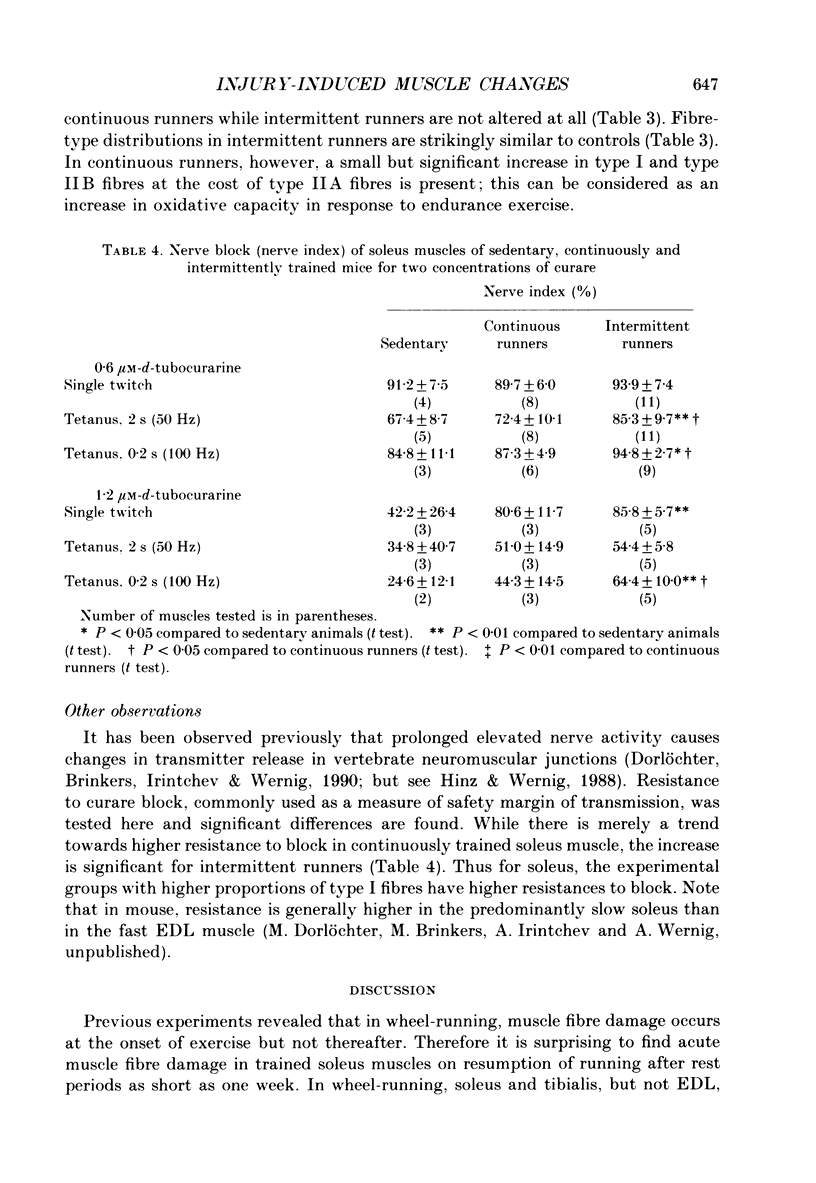





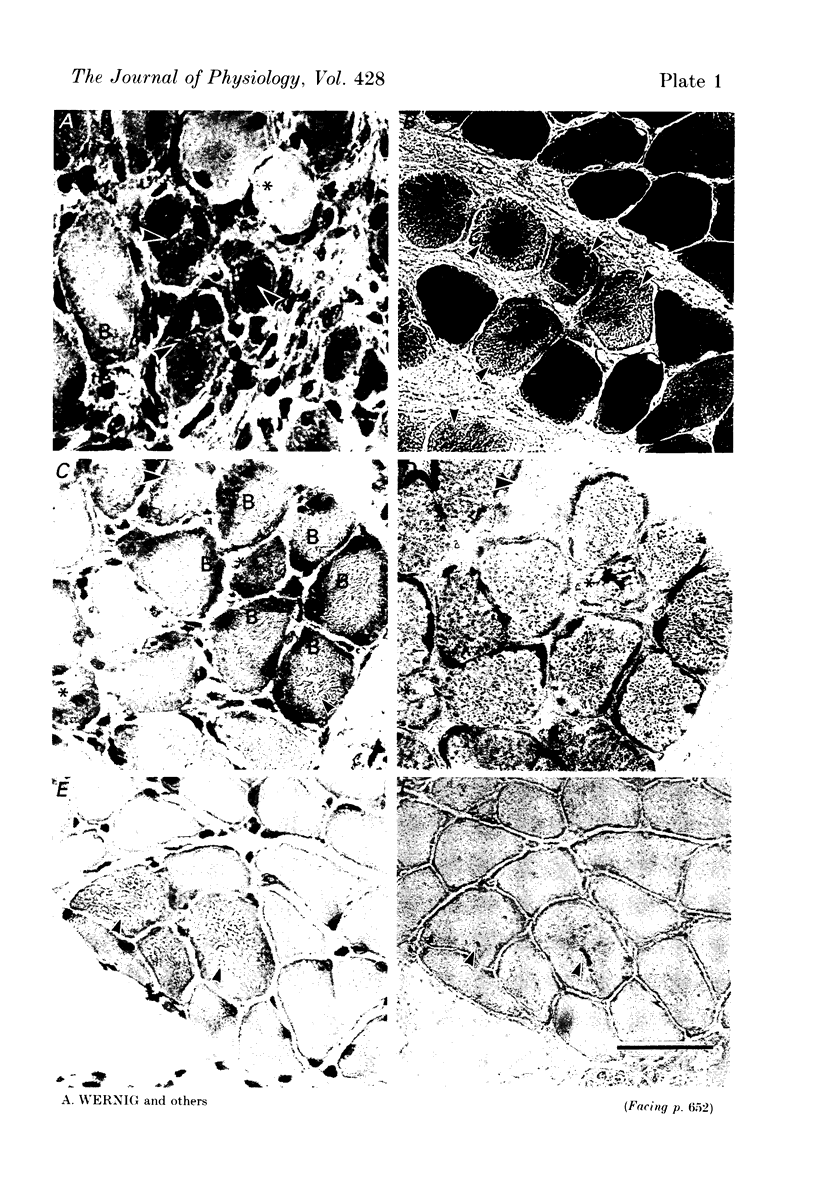
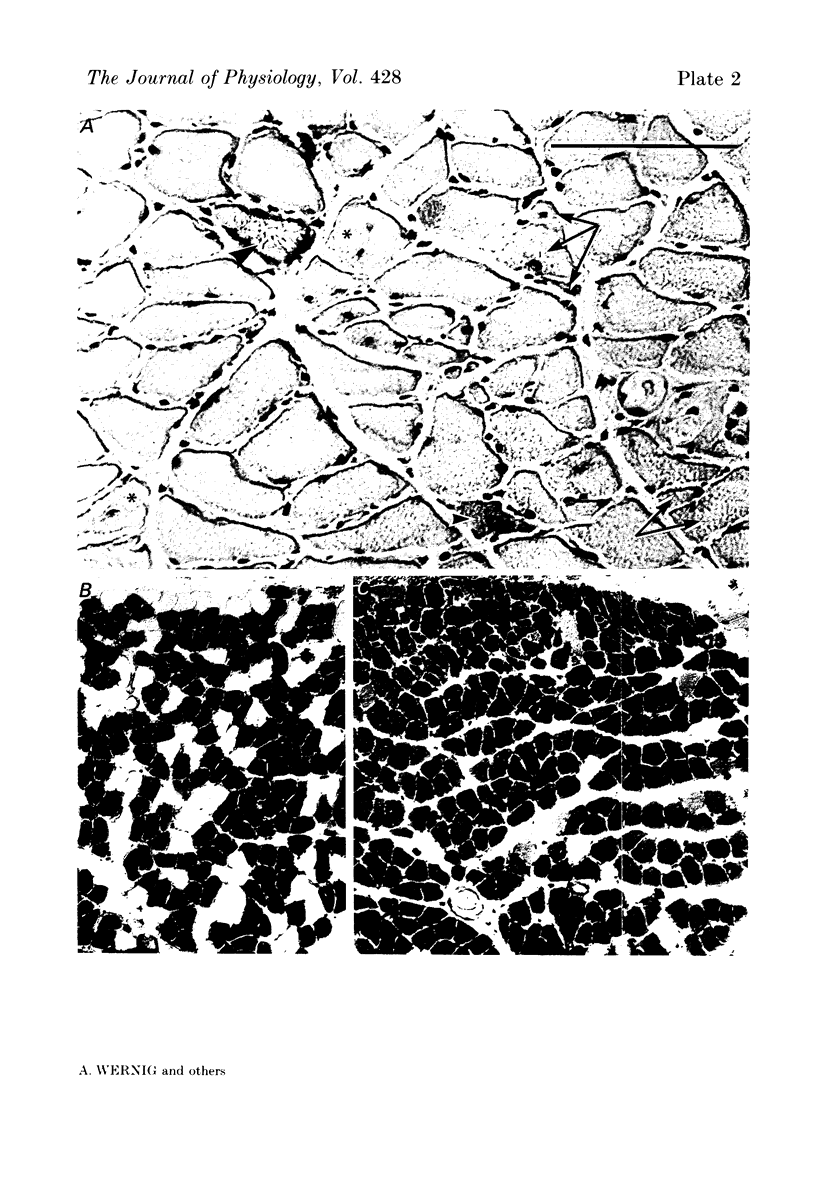
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. E., Bressler B. H., Ovalle W. K. Functional regeneration in the hindlimb skeletal muscle of the mdx mouse. J Muscle Res Cell Motil. 1988 Dec;9(6):499–515. doi: 10.1007/BF01738755. [DOI] [PubMed] [Google Scholar]
- Armstrong R. B., Ogilvie R. W., Schwane J. A. Eccentric exercise-induced injury to rat skeletal muscle. J Appl Physiol Respir Environ Exerc Physiol. 1983 Jan;54(1):80–93. doi: 10.1152/jappl.1983.54.1.80. [DOI] [PubMed] [Google Scholar]
- Badke A., Irintchev A. P., Wernig A. Maturation of transmission in reinnervated mouse soleus muscle. Muscle Nerve. 1989 Jul;12(7):580–586. doi: 10.1002/mus.880120709. [DOI] [PubMed] [Google Scholar]
- Butler J., Cosmos E. Enzymic markers to identify muscle-nerve formation during embryogenesis: modified myosin ATPase and silver-cholinesterase histochemical reactions. Exp Neurol. 1981 Sep;73(3):831–836. doi: 10.1016/0014-4886(81)90217-x. [DOI] [PubMed] [Google Scholar]
- Byrnes W. C., Clarkson P. M., White J. S., Hsieh S. S., Frykman P. N., Maughan R. J. Delayed onset muscle soreness following repeated bouts of downhill running. J Appl Physiol (1985) 1985 Sep;59(3):710–715. doi: 10.1152/jappl.1985.59.3.710. [DOI] [PubMed] [Google Scholar]
- Carlson B. M., Faulkner J. A. The regeneration of skeletal muscle fibers following injury: a review. Med Sci Sports Exerc. 1983;15(3):187–198. [PubMed] [Google Scholar]
- Darr K. C., Schultz E. Exercise-induced satellite cell activation in growing and mature skeletal muscle. J Appl Physiol (1985) 1987 Nov;63(5):1816–1821. doi: 10.1152/jappl.1987.63.5.1816. [DOI] [PubMed] [Google Scholar]
- Desypris G., Parry D. J. Relative efficacy of slow and fast alpha-motoneurons to reinnervate mouse soleus muscle. Am J Physiol. 1990 Jan;258(1 Pt 1):C62–C70. doi: 10.1152/ajpcell.1990.258.1.C62. [DOI] [PubMed] [Google Scholar]
- Edström L., Grimby L. Effect of exercise on the motor unit. Muscle Nerve. 1986 Feb;9(2):104–126. doi: 10.1002/mus.880090203. [DOI] [PubMed] [Google Scholar]
- Fridén J., Sfakianos P. N., Hargens A. R. Muscle soreness and intramuscular fluid pressure: comparison between eccentric and concentric load. J Appl Physiol (1985) 1986 Dec;61(6):2175–2179. doi: 10.1152/jappl.1986.61.6.2175. [DOI] [PubMed] [Google Scholar]
- Gonyea W. J., Sale D. G., Gonyea F. B., Mikesky A. Exercise induced increases in muscle fiber number. Eur J Appl Physiol Occup Physiol. 1986;55(2):137–141. doi: 10.1007/BF00714995. [DOI] [PubMed] [Google Scholar]
- Gorza L., Gundersen K., Lømo T., Schiaffino S., Westgaard R. H. Slow-to-fast transformation of denervated soleus muscles by chronic high-frequency stimulation in the rat. J Physiol. 1988 Aug;402:627–649. doi: 10.1113/jphysiol.1988.sp017226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth L., Samaha F. J. Procedure for the histochemical demonstration of actomyosin ATPase. Exp Neurol. 1970 Aug;28(2):365–367. [PubMed] [Google Scholar]
- Hikida R. S., Staron R. S., Hagerman F. C., Sherman W. M., Costill D. L. Muscle fiber necrosis associated with human marathon runners. J Neurol Sci. 1983 May;59(2):185–203. doi: 10.1016/0022-510x(83)90037-0. [DOI] [PubMed] [Google Scholar]
- Hinz I., Wernig A. Prolonged nerve stimulation causes changes in transmitter release at the frog neuromuscular junction. J Physiol. 1988 Jul;401:557–565. doi: 10.1113/jphysiol.1988.sp017179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irintchev A., Wernig A. Muscle damage and repair in voluntarily running mice: strain and muscle differences. Cell Tissue Res. 1987 Sep;249(3):509–521. doi: 10.1007/BF00217322. [DOI] [PubMed] [Google Scholar]
- Jones D. A., Newham D. J., Round J. M., Tolfree S. E. Experimental human muscle damage: morphological changes in relation to other indices of damage. J Physiol. 1986 Jun;375:435–448. doi: 10.1113/jphysiol.1986.sp016126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernell D., Eerbeek O., Verhey B. A., Donselaar Y. Effects of physiological amounts of high- and low-rate chronic stimulation on fast-twitch muscle of the cat hindlimb. I. Speed- and force-related properties. J Neurophysiol. 1987 Sep;58(3):598–613. doi: 10.1152/jn.1987.58.3.598. [DOI] [PubMed] [Google Scholar]
- Kuipers H., Drukker J., Frederik P. M., Geurten P., van Kranenburg G. Muscle degeneration after exercise in rats. Int J Sports Med. 1983 Feb;4(1):45–51. doi: 10.1055/s-2008-1026015. [DOI] [PubMed] [Google Scholar]
- Maier A., Gambke B., Pette D. Degeneration-regeneration as a mechanism contributing to the fast to slow conversion of chronically stimulated fast-twitch rabbit muscle. Cell Tissue Res. 1986;244(3):635–643. doi: 10.1007/BF00212544. [DOI] [PubMed] [Google Scholar]
- Maki T., Korthals J. K., Prockop L. D. Distribution of muscle changes in experimental ischemic myopathy. Muscle Nerve. 1986 Jun;9(5):394–398. doi: 10.1002/mus.880090503. [DOI] [PubMed] [Google Scholar]
- NACHLAS M. M., TSOU K. C., DE SOUZA E., CHENG C. S., SELIGMAN A. M. Cytochemical demonstration of succinic dehydrogenase by the use of a new p-nitrophenyl substituted ditetrazole. J Histochem Cytochem. 1957 Jul;5(4):420–436. doi: 10.1177/5.4.420. [DOI] [PubMed] [Google Scholar]
- Pette D., Vrbová G. Neural control of phenotypic expression in mammalian muscle fibers. Muscle Nerve. 1985 Oct;8(8):676–689. doi: 10.1002/mus.880080810. [DOI] [PubMed] [Google Scholar]
- SONG S. K., SHIMADA N., ANDERSON P. J. ORTHOGONAL DIAMETERS IN THE ANALYSIS OF MUSCLE FIBRE SIZE AND FORM. Nature. 1963 Dec 21;200:1220–1221. doi: 10.1038/2001220a0. [DOI] [PubMed] [Google Scholar]
- Salminen A., Vihko V. Acid proteolytic capacity in mouse cardiac and skeletal muscles after prolonged submaximal exercise. Pflugers Arch. 1980 Dec;389(1):17–20. doi: 10.1007/BF00587923. [DOI] [PubMed] [Google Scholar]
- Salmons S., Sréter F. A. Significance of impulse activity in the transformation of skeletal muscle type. Nature. 1976 Sep 2;263(5572):30–34. doi: 10.1038/263030a0. [DOI] [PubMed] [Google Scholar]
- Salmons S., Vrbová G. The influence of activity on some contractile characteristics of mammalian fast and slow muscles. J Physiol. 1969 May;201(3):535–549. doi: 10.1113/jphysiol.1969.sp008771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S., Gorza L., Sartore S., Saggin L., Ausoni S., Vianello M., Gundersen K., Lømo T. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil. 1989 Jun;10(3):197–205. doi: 10.1007/BF01739810. [DOI] [PubMed] [Google Scholar]
- Schmalbruch H. Regenerated muscle fibers in Duchenne muscular dystrophy: a serial section study. Neurology. 1984 Jan;34(1):60–65. doi: 10.1212/wnl.34.1.60. [DOI] [PubMed] [Google Scholar]
- Schmitt H. P. Measurement of voluntary muscle fiber cross sections: a comparative study of different possible methods. Microsc Acta. 1976 Jan;77(5):427–440. [PubMed] [Google Scholar]
- Segal S. S., Faulkner J. A. Temperature-dependent physiological stability of rat skeletal muscle in vitro. Am J Physiol. 1985 Mar;248(3 Pt 1):C265–C270. doi: 10.1152/ajpcell.1985.248.3.C265. [DOI] [PubMed] [Google Scholar]
- Staron R. S., Pette D. Nonuniform myosin expression along single fibers of chronically stimulated and contralateral rabbit tibialis anterior muscles. Pflugers Arch. 1987 Jun;409(1-2):67–73. doi: 10.1007/BF00584751. [DOI] [PubMed] [Google Scholar]
- Termin A., Staron R. S., Pette D. Myosin heavy chain isoforms in histochemically defined fiber types of rat muscle. Histochemistry. 1989;92(6):453–457. doi: 10.1007/BF00524756. [DOI] [PubMed] [Google Scholar]
- Vihko V., Rantamäki J., Salminen A. Exhaustive physical exercise and acid hydrolase activity in mouse skeletal muscle. A histochemical study. Histochemistry. 1978 Sep 15;57(3):237–249. doi: 10.1007/BF00492083. [DOI] [PubMed] [Google Scholar]
- Wernig A., Herrera A. A. Sprouting and remodelling at the nerve-muscle junction. Prog Neurobiol. 1986;27(3):251–291. doi: 10.1016/0301-0082(86)90023-7. [DOI] [PubMed] [Google Scholar]