Abstract
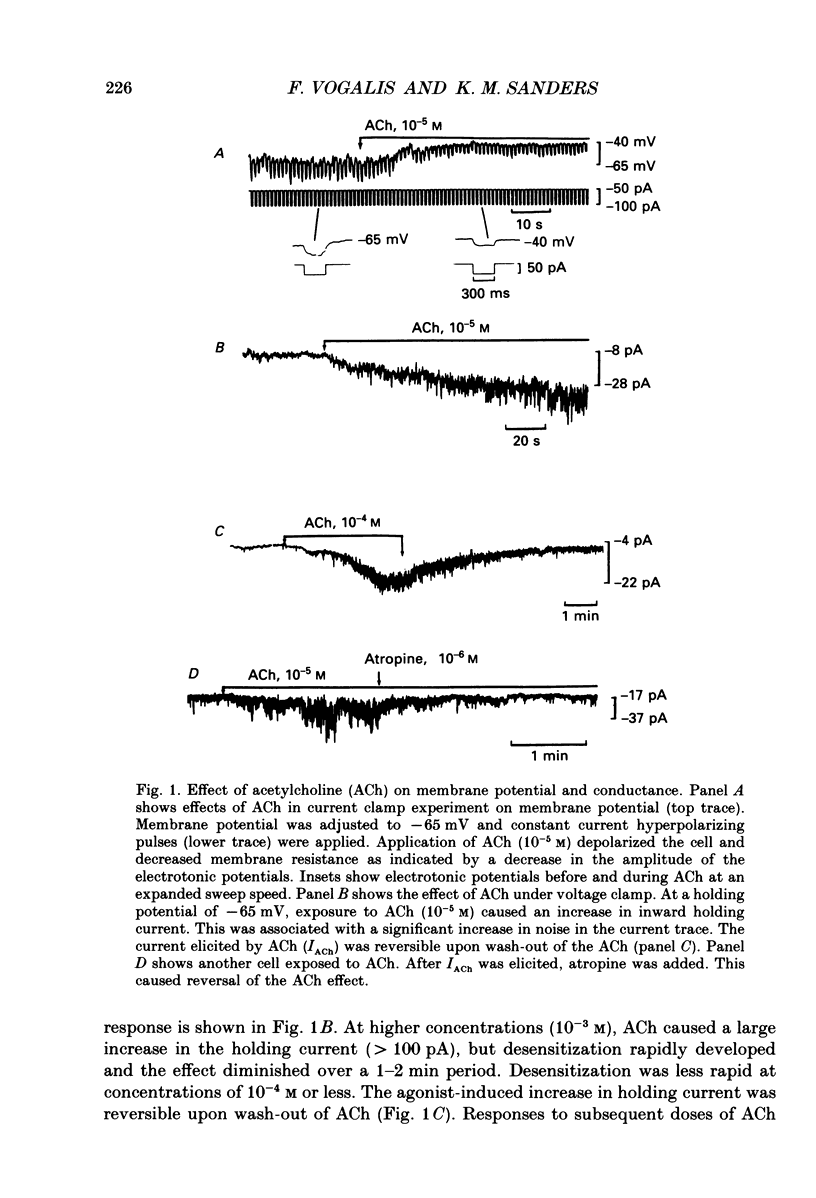
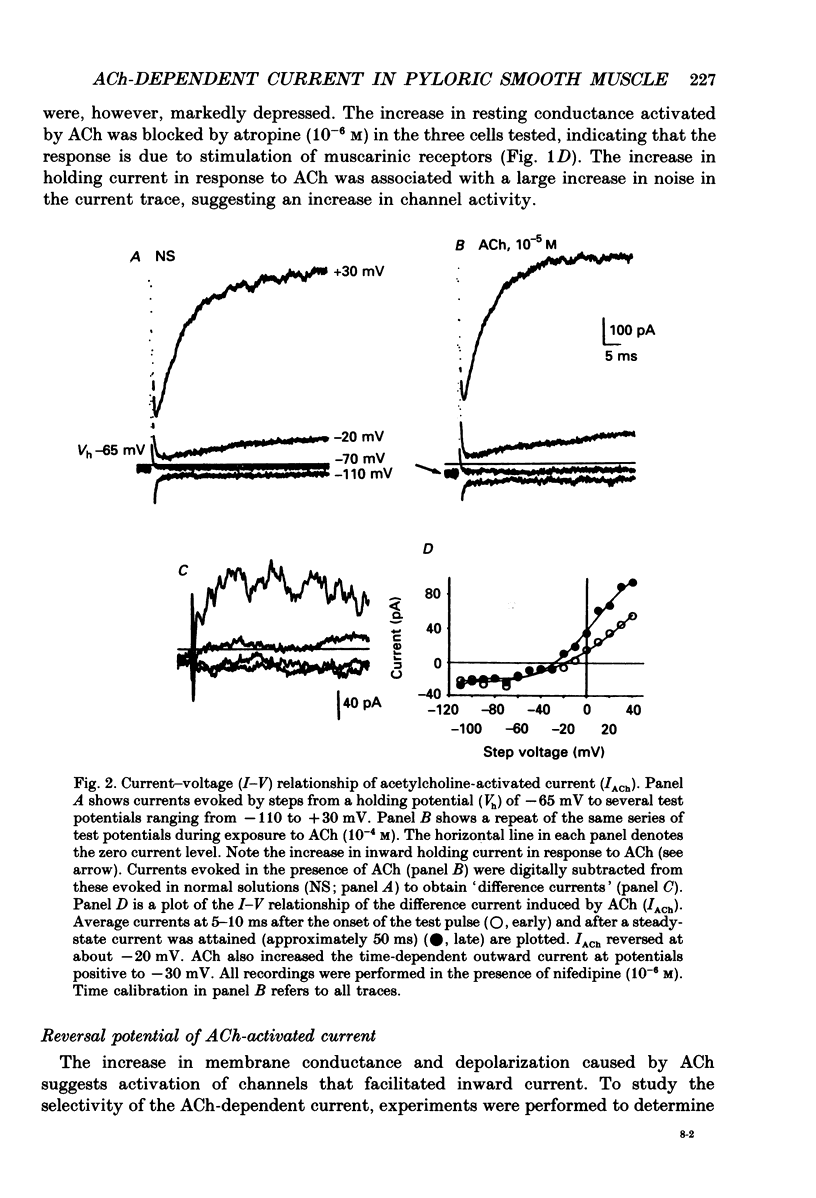
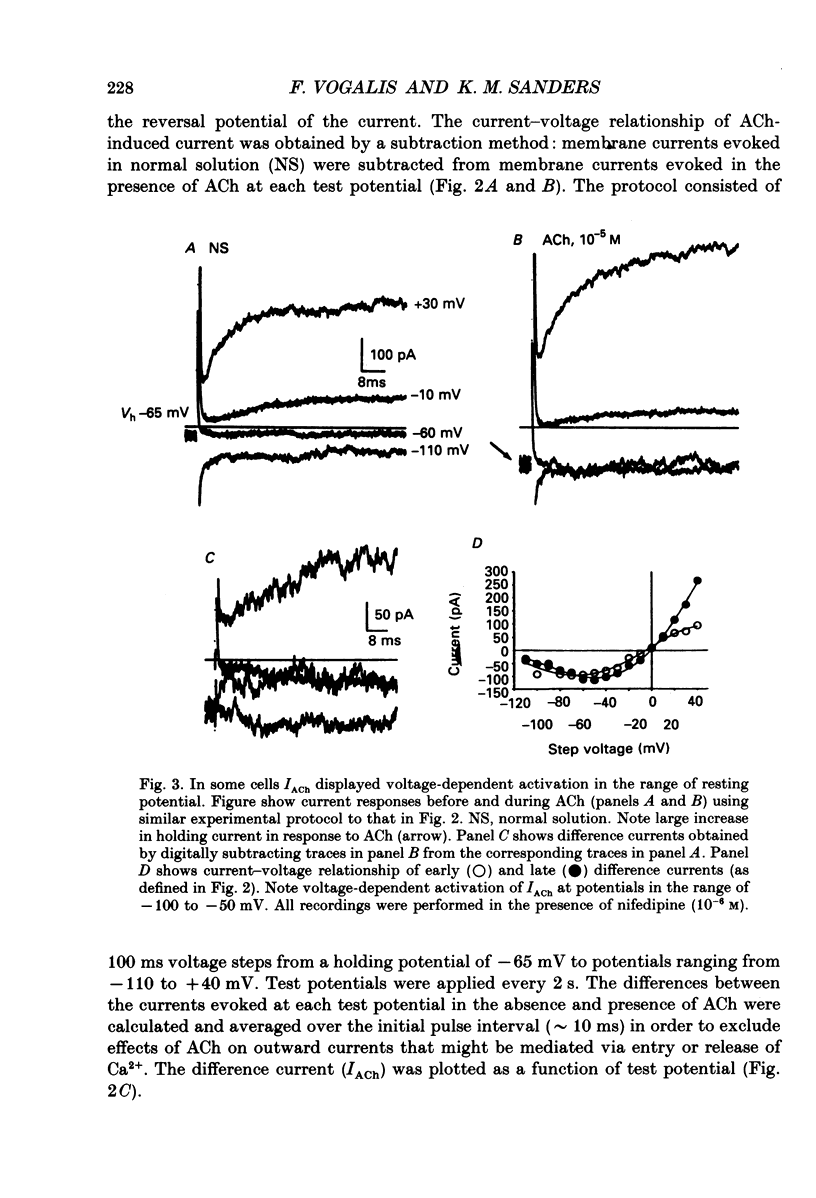
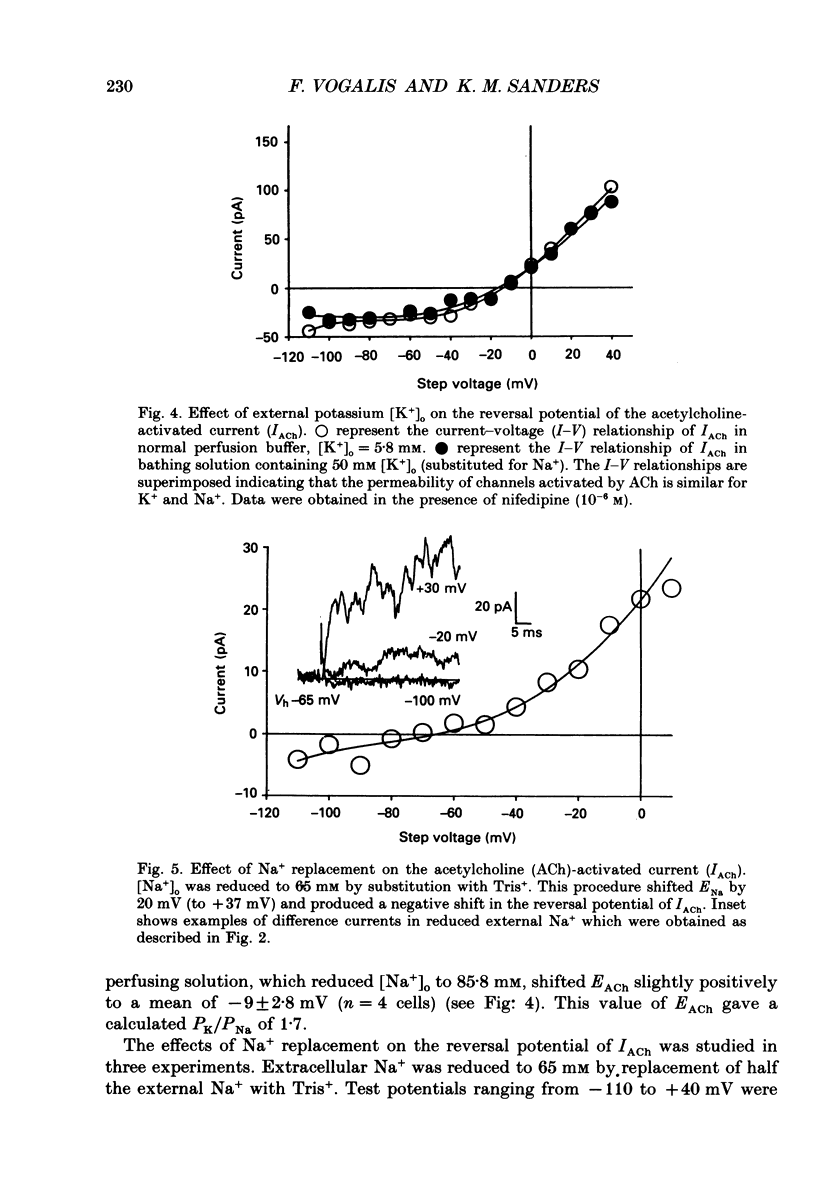
1. Cholinergic stimulation of circular muscle from the canine pyloric sphincter results in excitatory junction potentials and an increase in slow-wave frequency. Experiments were performed on isolated pyloric muscle cells to determine the effects of acetylcholine on membrane conductance and voltage-dependent ionic currents. 2. Acetylcholine depolarized circular muscle cells and increased membrane conductance. Under voltage clamp, these effects were associated with the development of an inward current. 3. The ACh-dependent current (IACh) reversed at about -20 mV and was about equally selective for potassium and sodium. Changes in the chloride gradient had no effect on the reversal potential of IACh. 4. The response to ACh was blocked by atropine suggesting that the response was mediated by muscarinic receptors. IACh could not be elicited in the presence of ions normally used to block potassium currents (e.g. bath-applied TEA+ and replacement of Ki+ with Csi+. 5. In some cells single-channel openings could be resolved in response to ACh. These channels had a slope conductance of 30 pS, and open probability increased with depolarization. 6. Acetylcholine had little or no effect on voltage-dependent Ca2+ currents, and increased voltage-dependent outward currents. The latter effect may have been due to increased release of Ca2+ from internal stores. 7. The non-selective cationic current elicited by ACh can explain the excitatory junction potentials in pyloric muscle cells that are generated by transmural nerve stimulation and may also explain the chronotropic effects of ACh on slow waves.
Full text
PDF



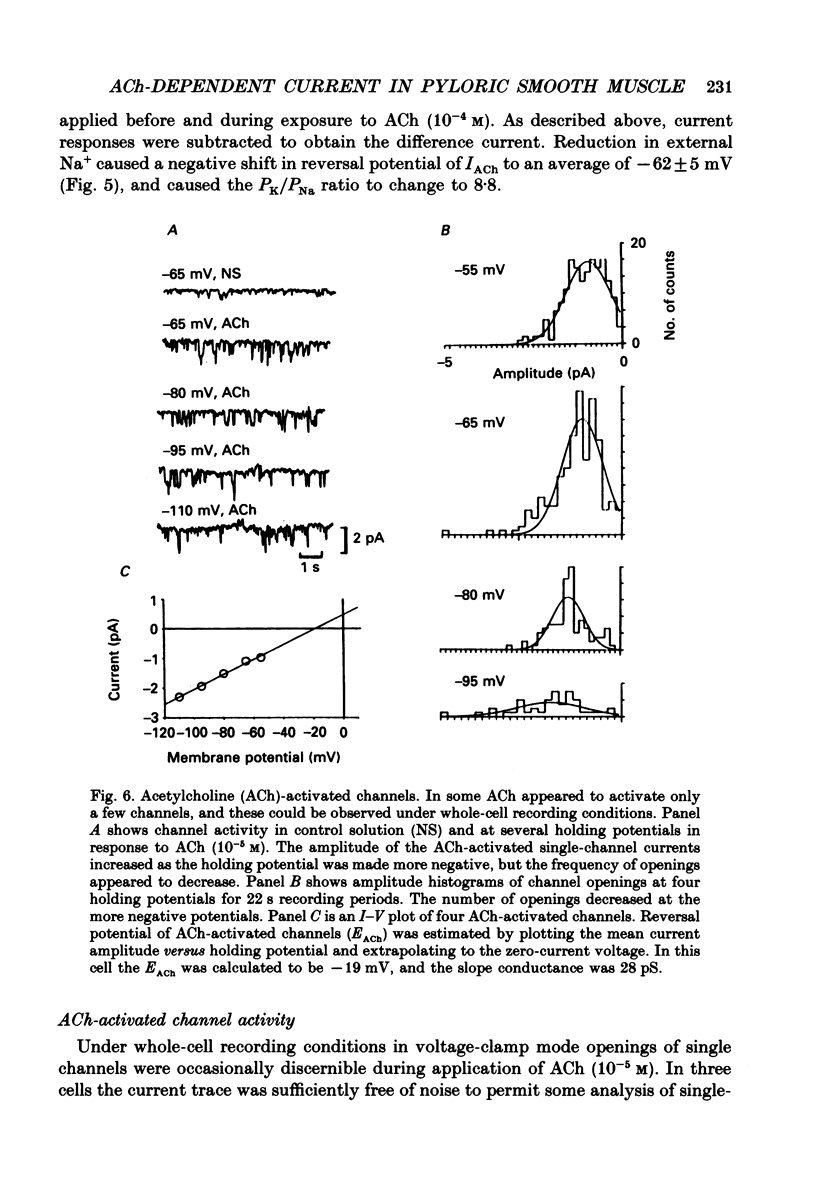
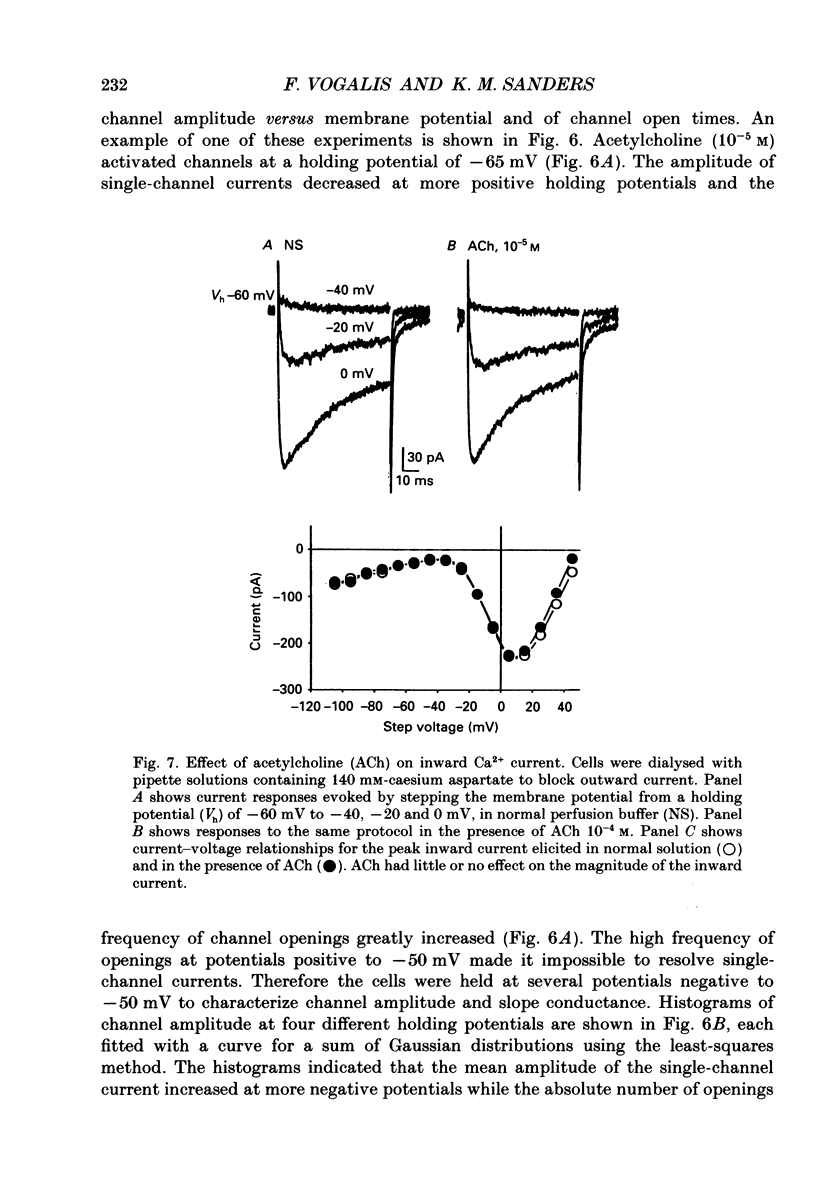




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Dwyer T. M., Hille B. The permeability of endplate channels to monovalent and divalent metal cations. J Gen Physiol. 1980 May;75(5):493–510. doi: 10.1085/jgp.75.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A. J., Sanders K. M. Gradient in excitation-contraction coupling in canine gastric antral circular muscle. J Physiol. 1985 Dec;369:283–294. doi: 10.1113/jphysiol.1985.sp015901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J. Acetylcholine activates an inward current in single mammalian smooth muscle cells. Nature. 1985 Jul 25;316(6026):345–347. doi: 10.1038/316345a0. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Clapp L. H., Vivaudou M. B., Walsh J. V., Jr, Singer J. J. Acetylcholine increases voltage-activated Ca2+ current in freshly dissociated smooth muscle cells. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2092–2096. doi: 10.1073/pnas.84.7.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole W. C., Carl A., Sanders K. M. Muscarinic suppression of Ca2+-dependent K current in colonic smooth muscle. Am J Physiol. 1989 Sep;257(3 Pt 1):C481–C487. doi: 10.1152/ajpcell.1989.257.3.C481. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R., Kitamura K., Kuriyama H. Acetylcholine activates single sodium channels in smooth muscle cells. Pflugers Arch. 1987 Sep;410(1-2):69–74. doi: 10.1007/BF00581898. [DOI] [PubMed] [Google Scholar]
- Langton P. D., Burke E. P., Sanders K. M. Participation of Ca currents in colonic electrical activity. Am J Physiol. 1989 Sep;257(3 Pt 1):C451–C460. doi: 10.1152/ajpcell.1989.257.3.C451. [DOI] [PubMed] [Google Scholar]
- Morgan K. G., Szurszewski J. H. Mechanisms of phasic and tonic actions of pentagastrin on canine gastric smooth muscle. J Physiol. 1980 Apr;301:229–242. doi: 10.1113/jphysiol.1980.sp013201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Publicover N. G., Sanders K. M. Effects of frequency on the wave form of propagated slow waves in canine gastric antral muscle. J Physiol. 1986 Feb;371:179–189. doi: 10.1113/jphysiol.1986.sp015967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders K. M., Smith T. K. Enteric neural regulation of slow waves in circular muscle of the canine proximal colon. J Physiol. 1986 Aug;377:297–313. doi: 10.1113/jphysiol.1986.sp016188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders K. M., Vogalis F. Organization of electrical activity in the canine pyloric canal. J Physiol. 1989 Sep;416:49–66. doi: 10.1113/jphysiol.1989.sp017748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims S. M., Singer J. J., Walsh J. V., Jr Cholinergic agonists suppress a potassium current in freshly dissociated smooth muscle cells of the toad. J Physiol. 1985 Oct;367:503–529. doi: 10.1113/jphysiol.1985.sp015837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. V., Bond M., Somlyo A. P., Scarpa A. Inositol trisphosphate-induced calcium release and contraction in vascular smooth muscle. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5231–5235. doi: 10.1073/pnas.82.15.5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogalis F., Sanders K. M. Excitatory and inhibitory neural regulation of canine pyloric smooth muscle. Am J Physiol. 1990 Jul;259(1 Pt 1):G125–G133. doi: 10.1152/ajpgi.1990.259.1.G125. [DOI] [PubMed] [Google Scholar]


