Abstract
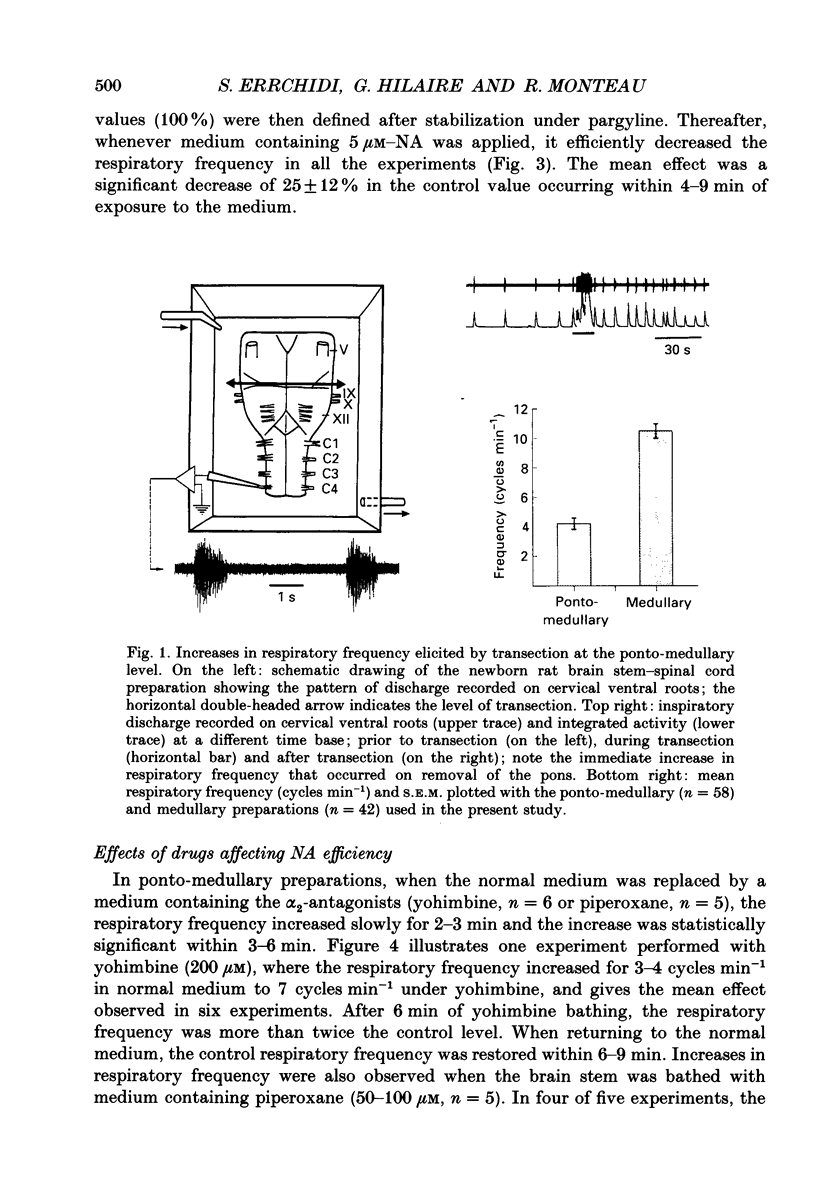
1. Respiratory activity was recorded on ventral cervical roots during in vitro experiments performed on superfused newborn rat brain stem-cervical cord preparations. 2. Eliminating the pontine structures by performing a transection at the level of the ponto-medullary junction resulted in a sustained increase in respiratory frequency, which suggests the existence of a pontine inhibitory drive impinging on the medullary rhythm generator. 3. Noradrenaline (NA) and drugs affecting NA efficiency were added to the bathing medium and the resulting changes in respiratory frequency were analysed. NA decreased the respiratory frequency, and this effect was potentiated by pargyline (an inhibitor of the NA degradation by monoamine oxidases) and blocked by yohimbine (an alpha 2-antagonist). 4. Yohimbine or piperoxane (which blocks the alpha 2-adrenoceptors) increased the resting respiratory frequency to the level reached after ponto-medullary transection, whereas pargyline or desipramine (which potentiates NA efficiency) decreased the respiratory rate. Since these effects were no longer observed after elimination of the pons, it is suggested that a permanent release of endogenous NA by pontine areas may modulate the activity of the medullary respiratory rhythm generator. 5. When alpha-methyltyrosine (an inhibitor of NA biosynthesis) was applied to the pons, the respiratory frequency was increased, whereas when tyrosine (a precursor of NA) was applied, the respiratory frequency decreased. This decrease was enhanced by pargyline, suppressed by alpha-methyltyrosine and blocked by piperoxane. 6. To conclude, it is suggested that the mechanisms underlying NA biosynthesis (i) continue to function under these in vitro experimental conditions and (ii) are responsible for a permanent release of endogenous NA, which slows down the respiratory frequency. These results are discussed as regards the possibility that the medullary respiratory rhythm generator may be modulated via the noradrenergic area A5 in the newborn rat.
Full text
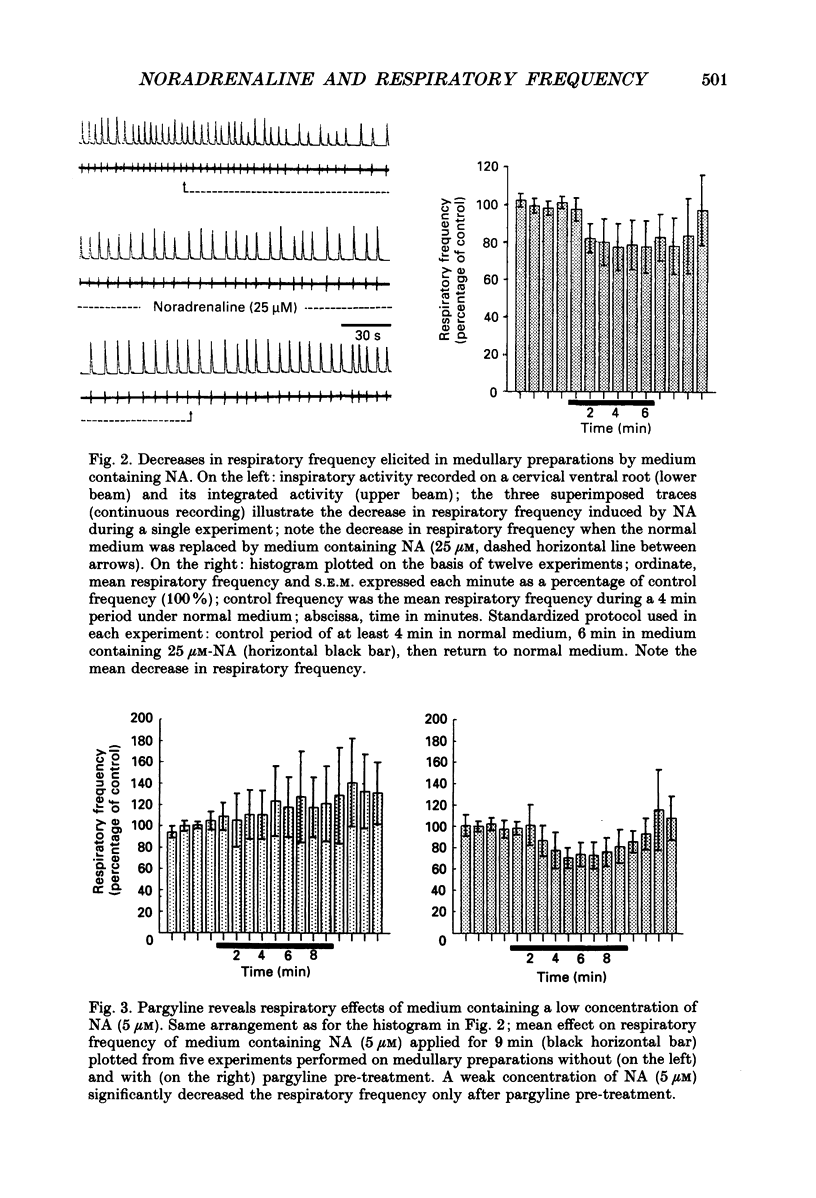
PDF


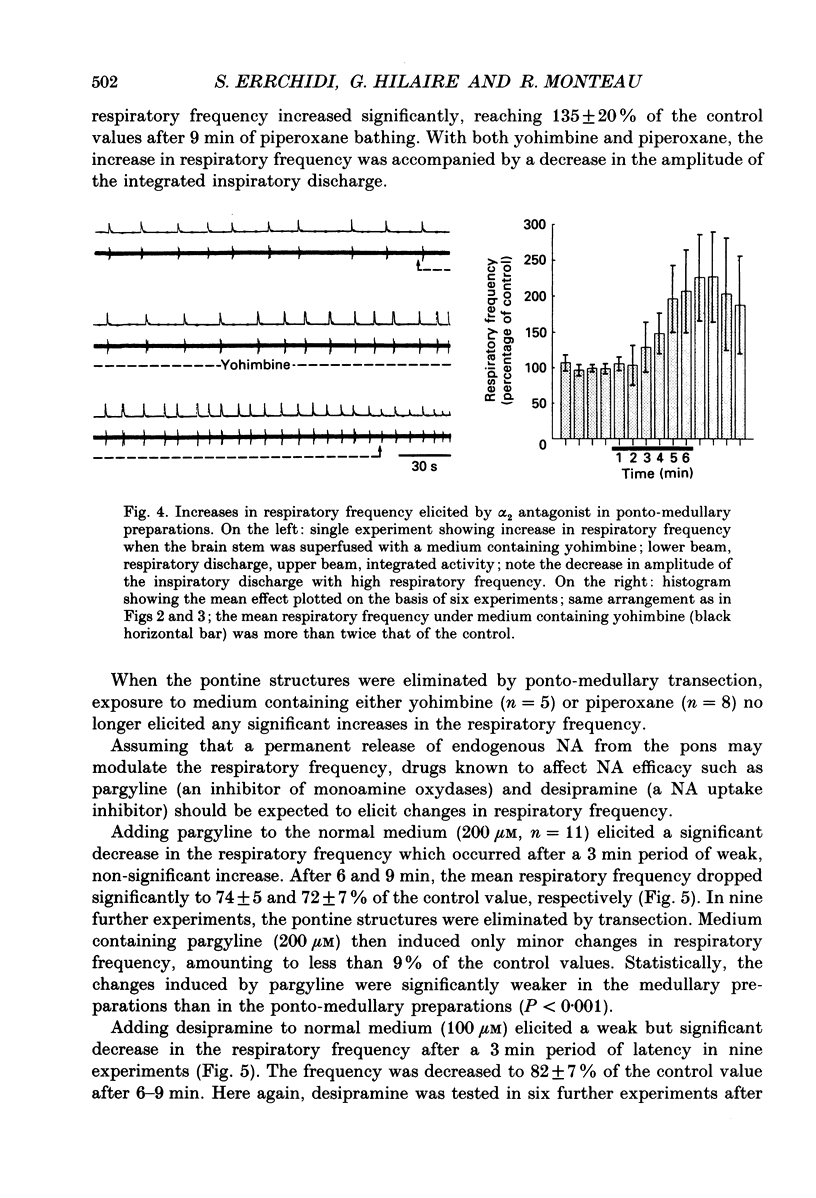
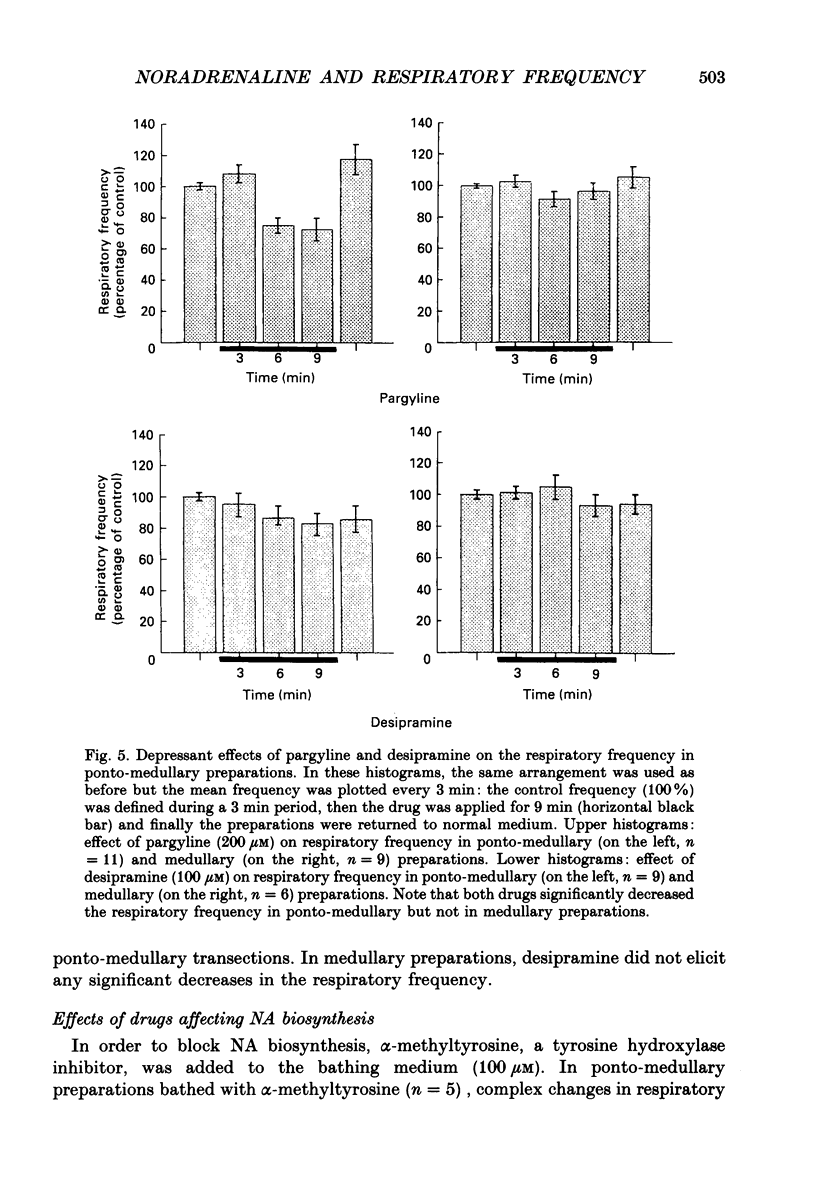
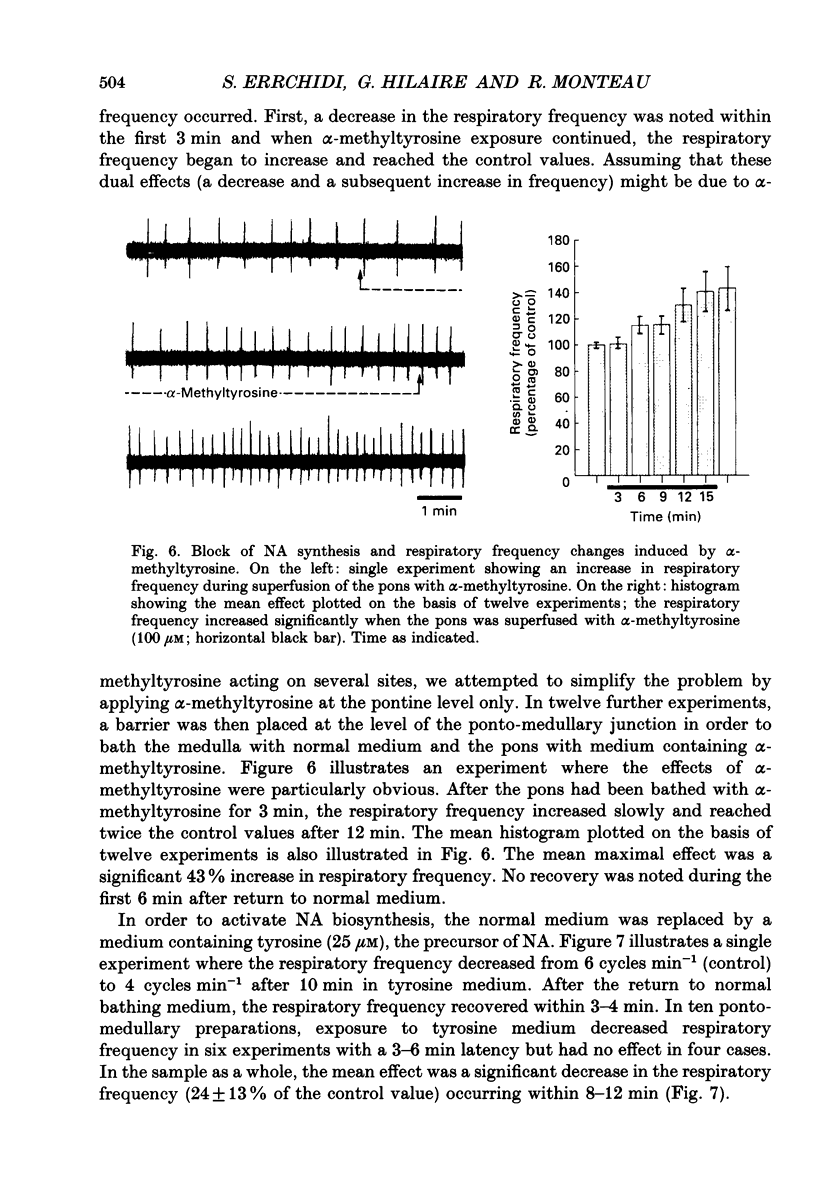
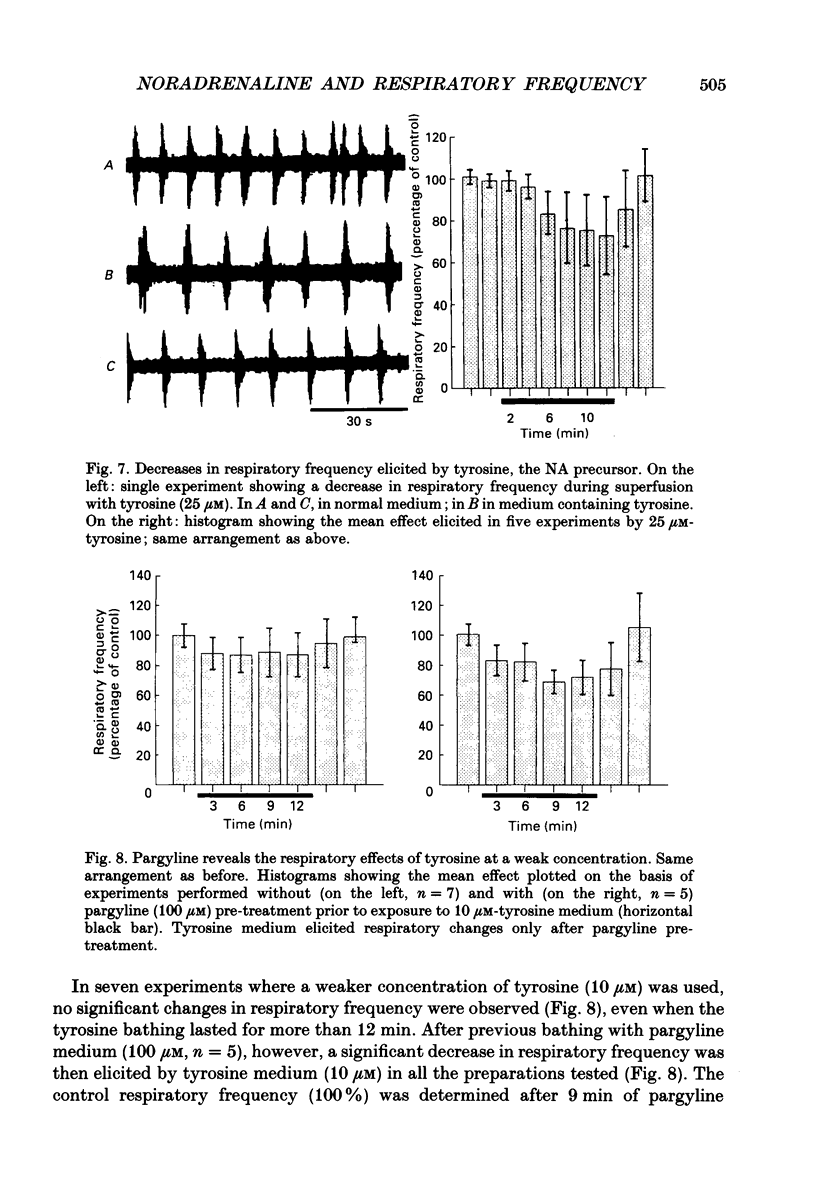





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaral D. G., Sinnamon H. M. The locus coeruleus: neurobiology of a central noradrenergic nucleus. Prog Neurobiol. 1977;9(3):147–196. doi: 10.1016/0301-0082(77)90016-8. [DOI] [PubMed] [Google Scholar]
- Andrade R., Aghajanian G. K. Single cell activity in the noradrenergic A-5 region: responses to drugs and peripheral manipulations of blood pressure. Brain Res. 1982 Jun 17;242(1):125–135. doi: 10.1016/0006-8993(82)90502-9. [DOI] [PubMed] [Google Scholar]
- Bolme P., Corrodi H., Fuxe K., Hökfelt T., Lidbrink P., Goldstein M. Possible involvement of central adrenaline neurons in vasomotor and respiratory control. Studies with clonidine and its interactions with piperoxane and yohimbine. Eur J Pharmacol. 1974 Sep;28(1):89–94. doi: 10.1016/0014-2999(74)90116-2. [DOI] [PubMed] [Google Scholar]
- Bolme P., Fuxe K. Pharmacological studies on a possible role of central noradrenaline neurons in respiratory control. J Pharm Pharmacol. 1973 Apr;25(4):351–352. doi: 10.1111/j.2042-7158.1973.tb10027.x. [DOI] [PubMed] [Google Scholar]
- Byrum C. E., Guyenet P. G. Afferent and efferent connections of the A5 noradrenergic cell group in the rat. J Comp Neurol. 1987 Jul 22;261(4):529–542. doi: 10.1002/cne.902610406. [DOI] [PubMed] [Google Scholar]
- Champagnat J., Denavit-Saubié M., Henry J. L., Leviel V. Catecholaminergic depressant effects on bulbar respiratory mechanisms. Brain Res. 1979 Jan 5;160(1):57–68. doi: 10.1016/0006-8993(79)90600-0. [DOI] [PubMed] [Google Scholar]
- Close J. M., Neil J. J., Loewy A. D. Actions of N-methyl aspartate and its antagonist aminophosphonovalerate on the A5 catecholamine cell group in rat. Brain Res. 1982 Oct 14;249(2):393–396. doi: 10.1016/0006-8993(82)90076-2. [DOI] [PubMed] [Google Scholar]
- Eldridge F. L., Millhorn D. E. Central regulation of respiration by endogenous neurotransmitters and neuromodulators. Annu Rev Physiol. 1981;43:121–135. doi: 10.1146/annurev.ph.43.030181.001005. [DOI] [PubMed] [Google Scholar]
- Foote S. L., Bloom F. E., Aston-Jones G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev. 1983 Jul;63(3):844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- GLOWINSKI J., AXELROD J. EFFECT OF DRUGS ON THE UPTAKE, RELEASE, AND METABOLISM OF H3-NOREPINEPHRINE IN THE RAT BRAIN. J Pharmacol Exp Ther. 1965 Jul;149:43–49. [PubMed] [Google Scholar]
- Guyenet P. G. Baroreceptor-mediated inhibition of A5 noradrenergic neurons. Brain Res. 1984 Jun 11;303(1):31–40. doi: 10.1016/0006-8993(84)90207-5. [DOI] [PubMed] [Google Scholar]
- Harada Y., Kuno M., Wang Y. Z. Differential effects of carbon dioxide and pH on central chemoreceptors in the rat in vitro. J Physiol. 1985 Nov;368:679–693. doi: 10.1113/jphysiol.1985.sp015883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada Y., Wang Y. Z., Kuno M. Central chemosensitivity to H+ and CO2 in the rat respiratory center in vitro. Brain Res. 1985 May 6;333(2):336–339. doi: 10.1016/0006-8993(85)91588-4. [DOI] [PubMed] [Google Scholar]
- Hilaire G., Monteau R., Errchidi S. Possible modulation of the medullary respiratory rhythm generator by the noradrenergic A5 area: an in vitro study in the newborn rat. Brain Res. 1989 Apr 24;485(2):325–332. doi: 10.1016/0006-8993(89)90577-5. [DOI] [PubMed] [Google Scholar]
- Hilaire G., Monteau R., Gauthier P., Rega P., Morin D. Functional significance of the dorsal respiratory group in adult and newborn rats: in vivo and in vitro studies. Neurosci Lett. 1990 Mar 26;111(1-2):133–138. doi: 10.1016/0304-3940(90)90357-f. [DOI] [PubMed] [Google Scholar]
- Kattwinkel J., Mars H., Fanaroff A. A., Klaus M. H. Urinary biogenic amines in idiopathic apnea of prematurity. J Pediatr. 1976 Jun;88(6):1003–1006. doi: 10.1016/s0022-3476(76)81063-3. [DOI] [PubMed] [Google Scholar]
- McMillen B. A., Shore P. A. The relative functional availability of brain noradrenaline and dopamine storage pools. J Pharm Pharmacol. 1977 Dec;29(12):780–781. doi: 10.1111/j.2042-7158.1977.tb11466.x. [DOI] [PubMed] [Google Scholar]
- McNamara M. C., Lawson E. E. Biogenic amine turnover in newborn and adult rabbit brainstem nuclei after pargyline administration. Respir Physiol. 1984 Dec;58(3):313–321. doi: 10.1016/0034-5687(84)90007-0. [DOI] [PubMed] [Google Scholar]
- Monteau R., Errchidi S., Gauthier P., Hilaire G., Rega P. Pneumotaxic centre and apneustic breathing: interspecies differences between rat and cat. Neurosci Lett. 1989 May 8;99(3):311–316. doi: 10.1016/0304-3940(89)90465-5. [DOI] [PubMed] [Google Scholar]
- Murakoshi T., Otsuka M. Respiratory reflexes in an isolated brainstem-lung preparation of the newborn rat: possible involvement of gamma-aminobutyric acid and glycine. Neurosci Lett. 1985 Nov 20;62(1):63–68. doi: 10.1016/0304-3940(85)90285-x. [DOI] [PubMed] [Google Scholar]
- Murakoshi T., Suzue T., Tamai S. A pharmacological study on respiratory rhythm in the isolated brainstem-spinal cord preparation of the newborn rat. Br J Pharmacol. 1985 Sep;86(1):95–104. doi: 10.1111/j.1476-5381.1985.tb09439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson L., Seiger A. Early prenatal ontogeny of central monoamine neurons in the rat: fluorescence histochemical observations. Z Anat Entwicklungsgesch. 1972;137(3):301–316. doi: 10.1007/BF00519099. [DOI] [PubMed] [Google Scholar]
- SPECTOR S., SJOERDSMA A., UDENFRIEND S. BLOCKADE OF ENDOGENOUS NOREPINEPHRINE SYNTHESIS BY ALPHA-METHYL-TYROSINE, AN INHIBITOR OF TYROSINE HYDROXYLASE. J Pharmacol Exp Ther. 1965 Jan;147:86–95. [PubMed] [Google Scholar]
- Saether K., Hilaire G., Monteau R. Dorsal and ventral respiratory groups of neurons in the medulla of the rat. Brain Res. 1987 Sep 1;419(1-2):87–96. doi: 10.1016/0006-8993(87)90571-3. [DOI] [PubMed] [Google Scholar]
- Spector S., Gordon R., Sjoerdsma A., Udenfriend S. End-product inhibition of tyrosine hydroxylase as a possible mechanism for regulation of norepinephrine synthesis. Mol Pharmacol. 1967 Nov;3(6):549–555. [PubMed] [Google Scholar]
- Suzue T. Respiratory rhythm generation in the in vitro brain stem-spinal cord preparation of the neonatal rat. J Physiol. 1984 Sep;354:173–183. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]


